Figure 7. A homodimer coordinates the pH-sensitive LRP2 molecular machine.
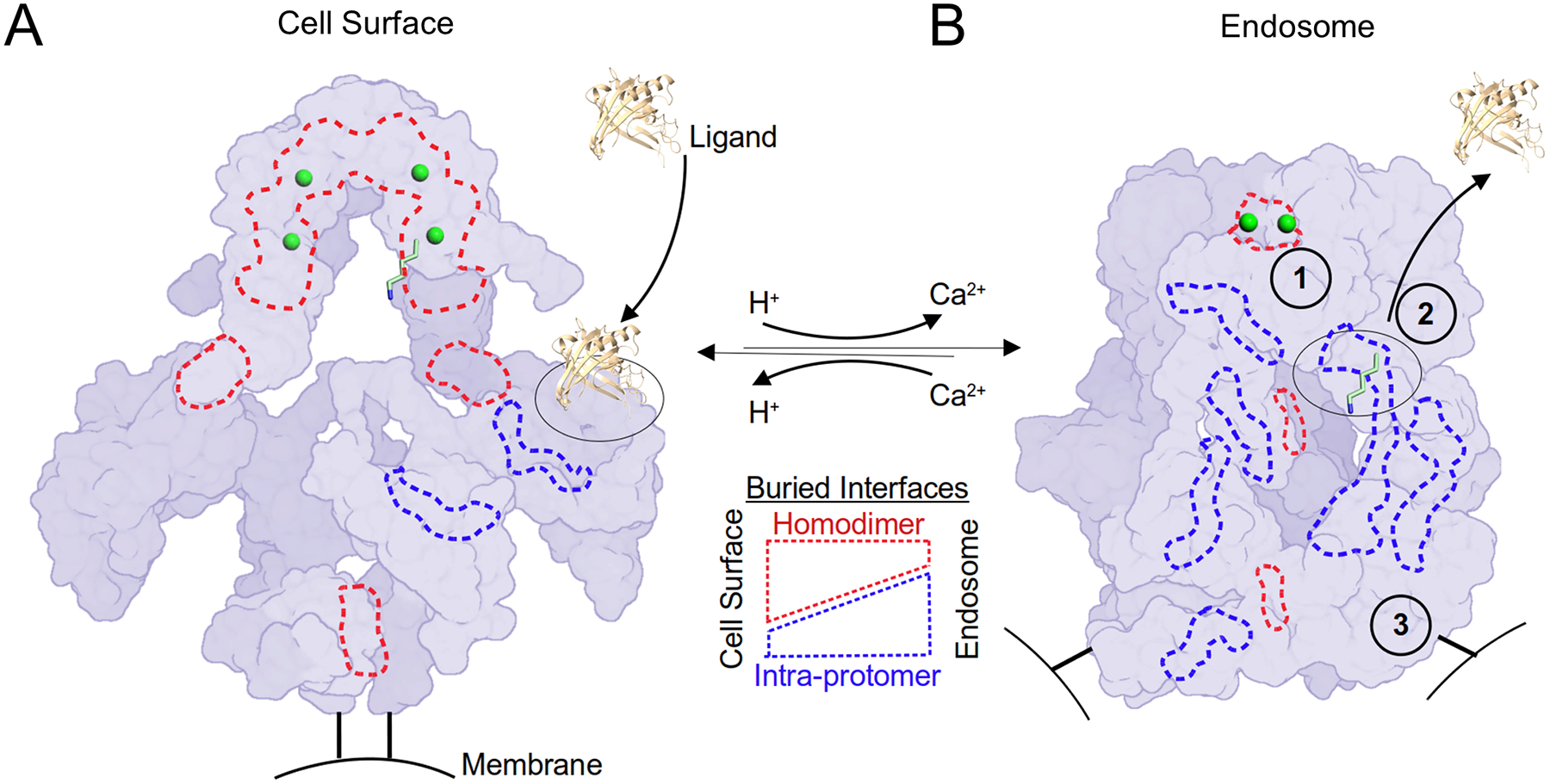
LRP2 is rendered as a schematic surface at extracellular and endosomal pH in the left and right panels respectively. Protomers are distinguished by two shades of purple. The C-termini of the extracellular domains that are directed towards the membrane insertions are rendered as black bars. Putative Ca2+ ions within the canopy are rendered as green spheres. K1430 is rendered in sticks with 6-fold magnification as one example of the 28 intramolecular ligands, and its binding partner, L8, is outlined with a dashed black oval. An extracellular ligand of ~20kDa rendered to scale is represented as a cartoon (PDB ID: 5JR8). Selected examples of buried surface area at the homodimer interfaces are outlined in dashed red lines, and intra-protomer interfaces are outlined in dashed blue lines. (A) At the cell surface, homodimer interfaces predominate. Hypothetical ligand binding to L8 is depicted. At the cell surface, the homodimer acts as a scaffold to keep L repeats open for ligand interactions and maintain distance between intramolecular ligands and their cognate L repeats. (B) In the endosomal compartment, 1) a pair of Ca2+ ions is released from the canopy and the homodimer interface unlocks to enable intramolecular ligands to interact with their cognate L repeats. 2) An intramolecular ligand displaces the extracellular ligand. As a result of these structural transitions, intra-protomer interfaces now predominate at endosomal pH. 3) The homodimer reorganization separates the membrane insertion points by >140 Å, which may serve as a compartment-specific trafficking signal.
See also Movie S3
