Figure 3. The N-Plug is Essential for Arginine-Enhanced Transport of Leucine by drSLC38A9.
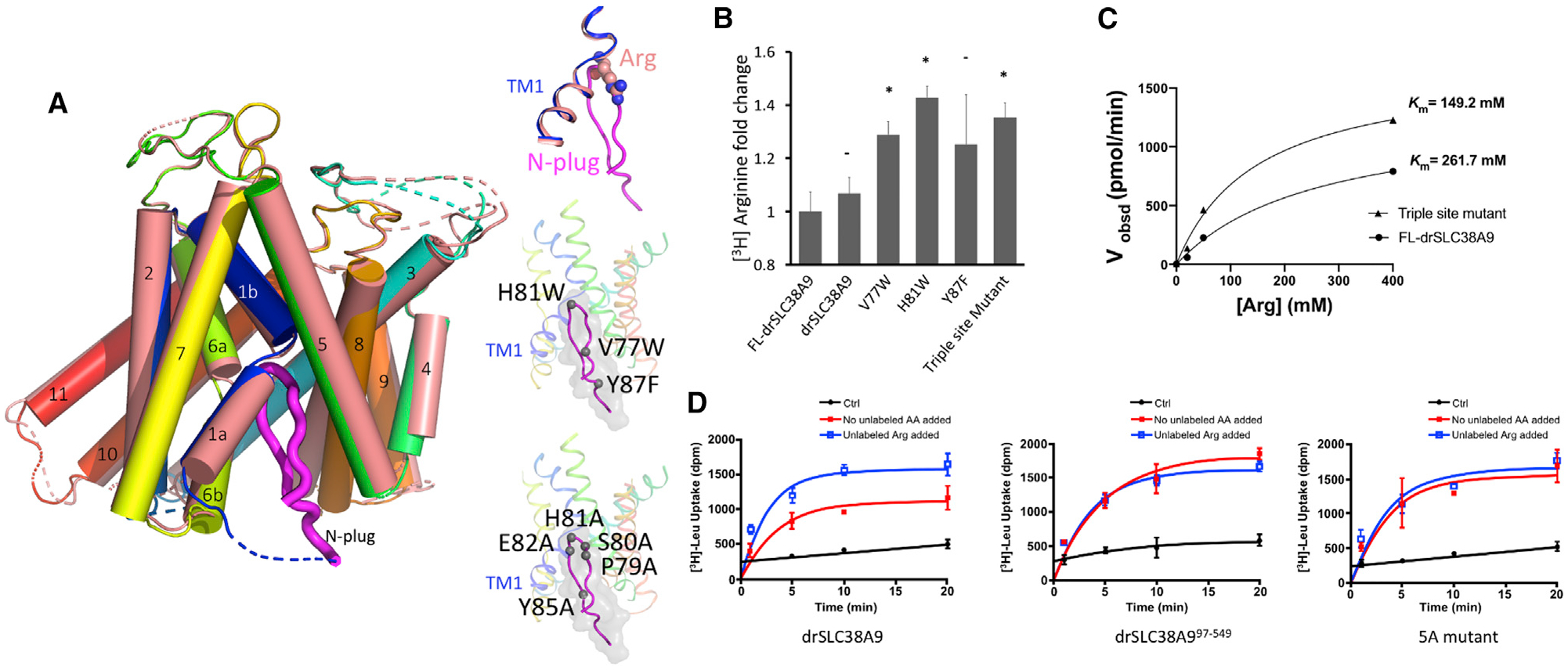
(A) Superposition of substrate binding site of the arginine-bound state (PDB: 6C08) with the N-plug inserted state of drSLC38A9. TM1s of two different states are shown in pink and blue. Atoms of arginine molecule are depicted as spheres while the N-plug is in magenta. Top inset: close-up view of Arg clashed on top of the N-plug in the superposition. Middle inset: positions of triple site mutations on the N-plug. Bottom inset: positions of 5A mutations on the N-plug.
(B) [3H]Arginine steady-state uptake by drSLC38A9 variants. Fold changes are relative to the uptake by full-length drSLC38A9, and bar graphs show mean ± SEM (n = 3, *p < 0.1).
(C) Michaelis-Menten plot for steady-state kinetic analysis of arginine uptake by triple site mutant and full-length drSLC38A9. The experiment was repeated more than three times with similar results and a representative one is shown. kcat values for FL-drSLC38A9 and the triple site mutant are 79 and 60 min−1.
(D) Adding 200 μM unlabeled arginine boosts leucine transport by drSLC38A9 in proteoliposomes (left). Either deletion (middle) or mutation of the N-plug (right) interferes with the arginine enhancement of leucine transport. The mutant proteins show similar transport capacity for leucine regardless of whether arginine was added.
