FIGURE 2.
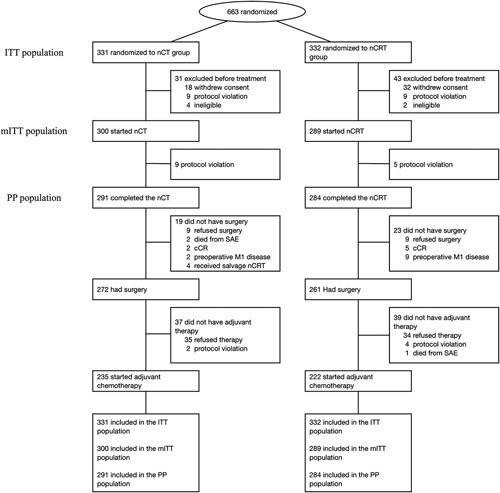
Trial profile. The ITT population comprised all patients who were were randomized to treatment. The mITT population comprised all patients who were randomized to treatment and received at least 1 dose of study treatment. The PP population comprised of patients who completed the neoadjuvant therapy without major protocol deviations. Ccr indicates clinical complete response; ITT, intention-to-treat; mITT, modified intention-to-treat; nCRT, neoadjuvant radiochemotherapy; nCT, neoadjuvant chemotherapy; PP, per-protocol; SAE, serious adverse event.
