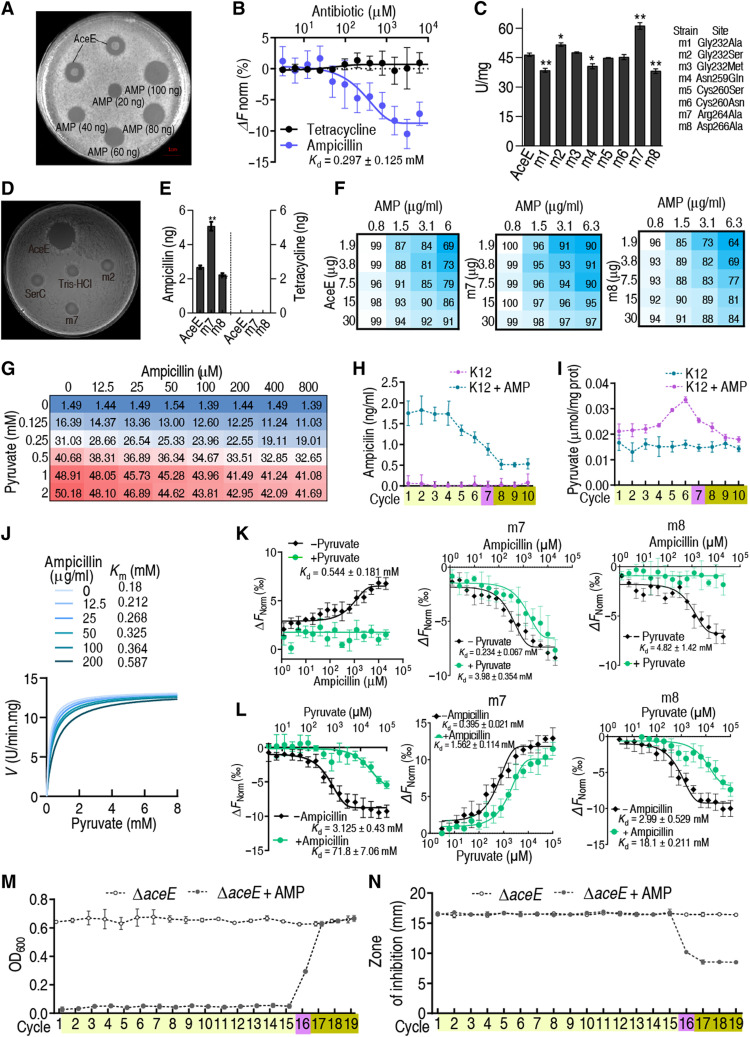
Fig. 3. AMP-binding capability of AceE and effect.
(A) Oxford cup test for the AMP-binding capability of AceE. Purified recombinant AceE was mixed with AMP, and then AceE was precipitated by acetone. The precipitated protein was diluted and used for the test. (B) MST for binding of AceE with AMP. Tetracycline was used as a control (n = 3). (C) Activity of PDH with point mutation on AceE (n = 3). (D) Oxford cup test for AMP binding to AceE with point mutation. (E) Quantification for AMP or tetracycline binding to recombinant proteins (n = 3). (F) Effect of AMP on the activity of AceE, m7, and m8. (G) Activity of PDH in the presence of AMP. (H) Intracellular AMP concentration during cyclic daily intermittent exposure to AMP (0.625 μg/ml) (see Materials and Methods for details) (n = 4). (I) Pyruvate level in E. coli K12 during cyclic daily intermittent exposure to AMP. (J) Michaelis-Menten kinetics of PDH in AMP (0 to 200 μg). (K and L) MST for competition between pyruvate and AMP with AceE, m7, and m8 (n = 4). (M) Growth/viability of the E. coli K12 ∆aceE after the indicated number of cycles exposed to AMP (n = 4). (N) MIC of (M) (n = 4). Experiments were performed the same as described in Fig. 1 (A, B, and F). Results are displayed as means ± SEM, and statistically significant differences are identified by Kruskal-Wallis followed by Dunn’s multiple comparison post hoc test unless otherwise indicated. *P < 0.05 and **P < 0.01.