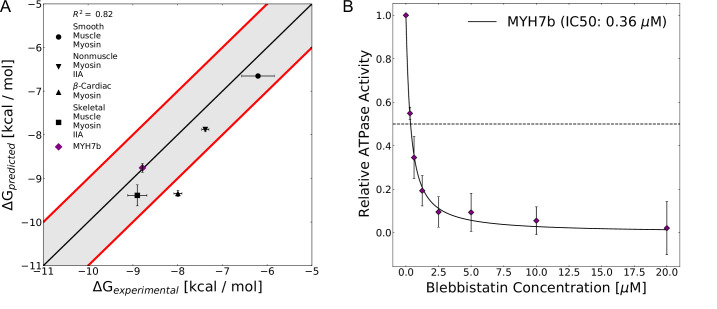
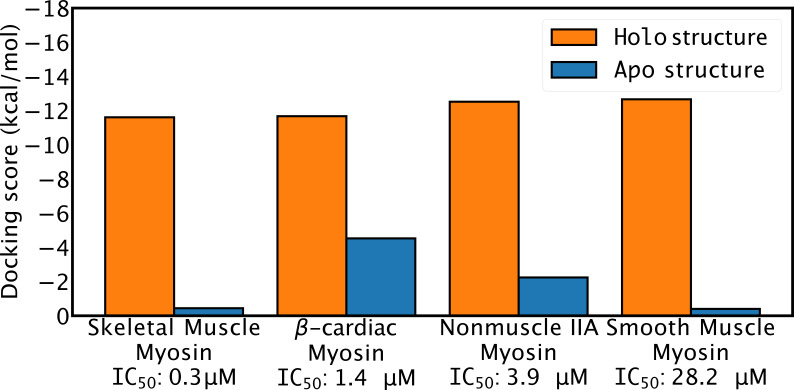
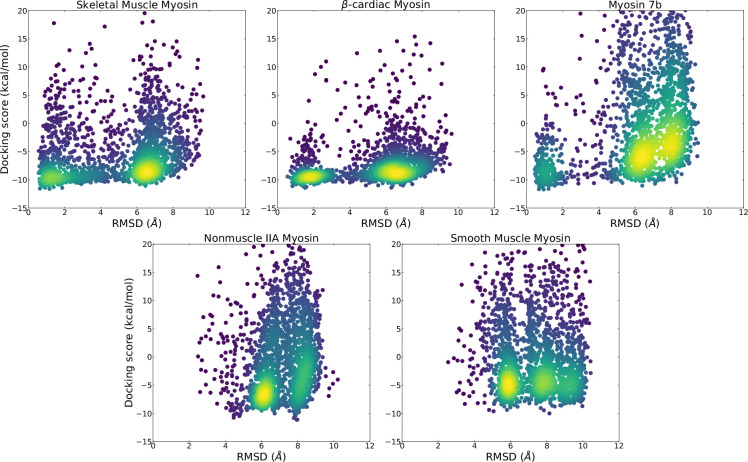
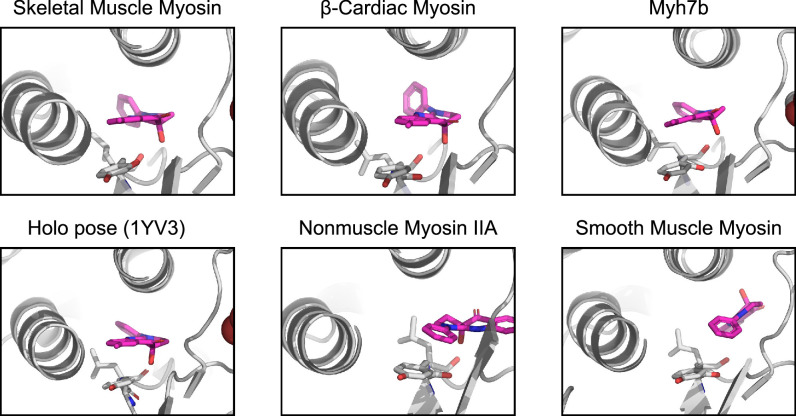
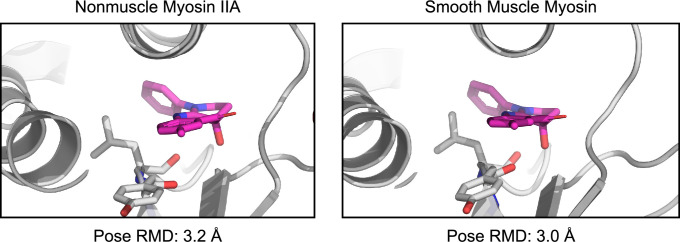
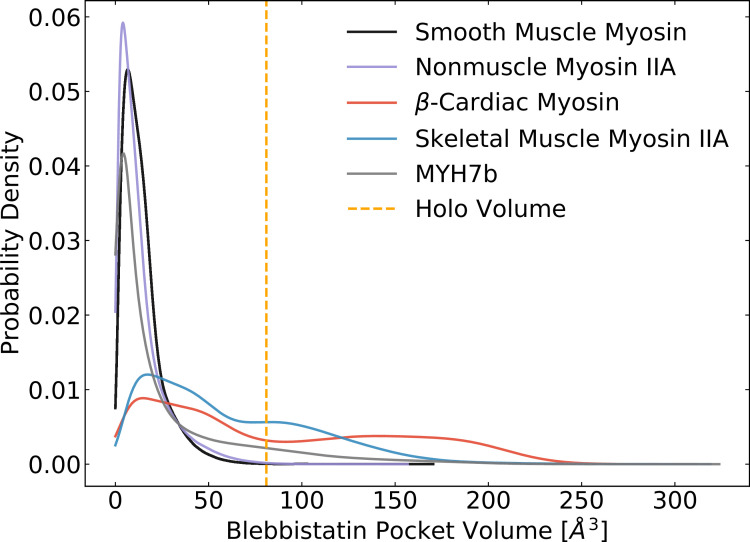
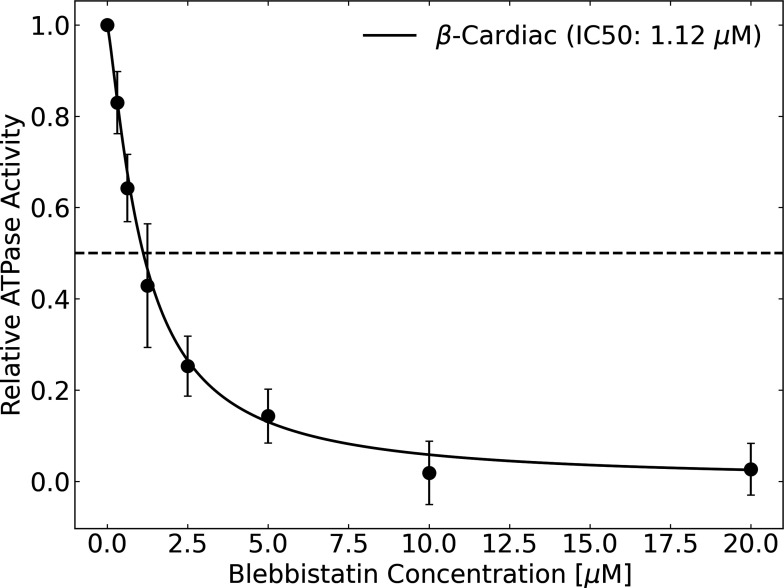
Figure 6. The computed free energy of binding for blebbistatin from MSM-docking accurately predicts binding free energies for existing experimental data and for a myosin isoform whose blebbistatin sensitivity was not known.
(A) Predictions from MSM-docking are highly correlated to experimental values (R2=0.82) and most predictions are within 1 kcal/mol of experimental values. Error bars for predicted free energies of binding represent bootstrapped estimate of standard error of the mean from 250 trials. Error bars for experimental values show the standard error of the IC50 or Ki converted to a binding free energy. (B) An NADH-linked ATPase assay indicates that MYH7b is highly sensitive to blebbistatin inhibition (IC50: 0.36 μM), consistent with the prediction from MSM-docking. Data show the mean ATPase activity ± standard deviation across 5 experimental replicates (2 biological replicates, each with two or three technical replicates).