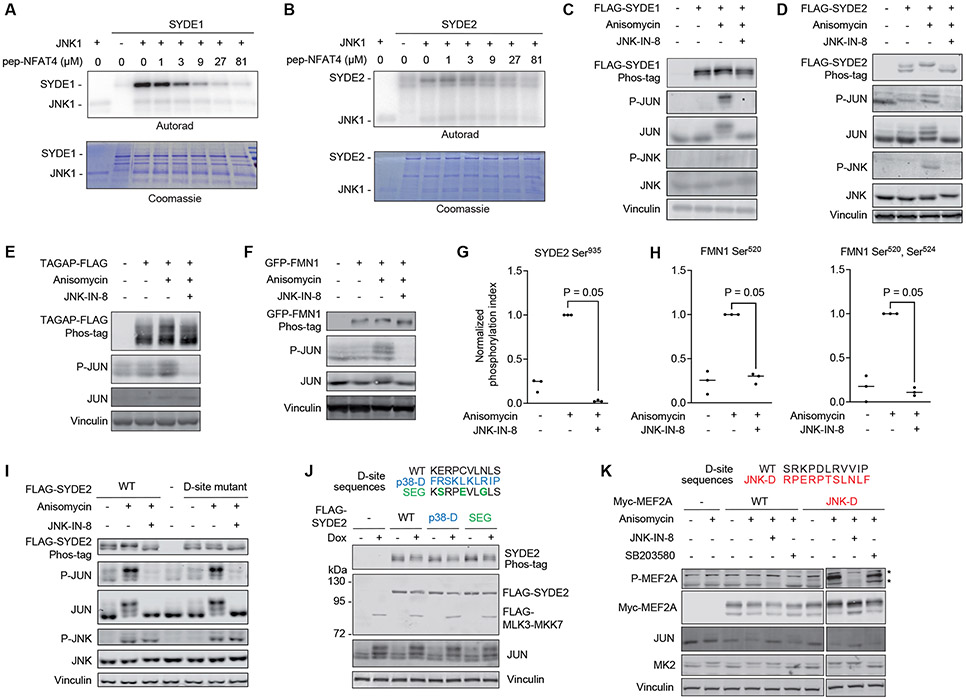
Figure 6. JNK substrate discovery.
(A) In vitro kinase assay measuring JNK1-mediated phosphorylation of full length SYDE1. Reactions were performed with increasing concentrations of the NFAT4 D-site competitor peptide (pep-NFAT4). Representative images from 3 separate experiments are shown. (B) In vitro kinase assay measuring JNK1-mediated phosphorylation of full length SYDE2, performed as in (A). Representative images from 3 separate experiments are shown. (C-F) Immunoblotting analysis with the indicated antibodies of HEK293T cells expressing FLAG-SYDE1, FLAG-SYDE2, FLAG-TAGAP, or GFP-FMN1 and treated with anisomycin following preincubation with or without the covalent JNK inhibitor JNK-IN-8. Lysates were subjected to either Phos-tag (top image) or standard (all others) SDS-PAGE. A representative immunoblot is shown for 2 (SYDE1), 3 (SYDE2), 5 (TAGAP) and 5 (FMN1) independent experiments. (G) LC-MS/MS analysis of tryptic peptides derived from immunopurified FLAG-SYDE2 transiently expressed in HEK293T cells treated as indicated. The graph shows the ratio of the Ser935-phosphorylated peptide to the corresponding unphosphorylated peptide. Data are normalized to the anisomycin treated sample. N=3 independent experiments. Significance was calculated using a one-tailed Mann-Whitney non-parametric test. (H) LC-MS/MS analysis of singly or doubly phosphorylated GFP-FMN1-derived tryptic phosphopeptides spanning Ser520 and Ser524 showing the ratio of phosphorylated to unphosphorylated peptides, normalized to the anisomycin treated sample. N=3 independent experiments. Significance was calculated using a one-tailed Mann-Whitney non-parametric test. (I) Immunoblotting analysis with the indicated antibodies of lysates from HEK293T cells expressing either WT SYDE2 or its D-site mutant (R1179Q/P1180Q/L1183A/L1185A) and treated with anisomycin following preincubation with or without the covalent JNK inhibitor JNK-IN-8. N=3 independent experiments. (J) Immunoblots of lysates from HEK293T cells harboring an episomal Dox-inducible MLK3-MKK7 expression plasmid and transfected with FLAG-SYDE2 variants in which the D-site was substituted with the sequence shown at top. Cells were incubated for 6 hr in the presence or absence of Dox prior to lysis. N=2 independent experiments. (K) Immunoblots of HEK293T cell lysates transfected with plasmids expressing either WT MEF2A or a mutant in which the D-site was exchanged for an optimal JNK-binding sequence shown at top (JNK-D). Cells were treated with anisomycin in the presence or absence of JNK-IN-8 or the p38α/β inhibitor SB203580 prior to lysis. Asterisk (*) indicates non-specific bands present in the untransfected control samples. N=2 independent experiments.