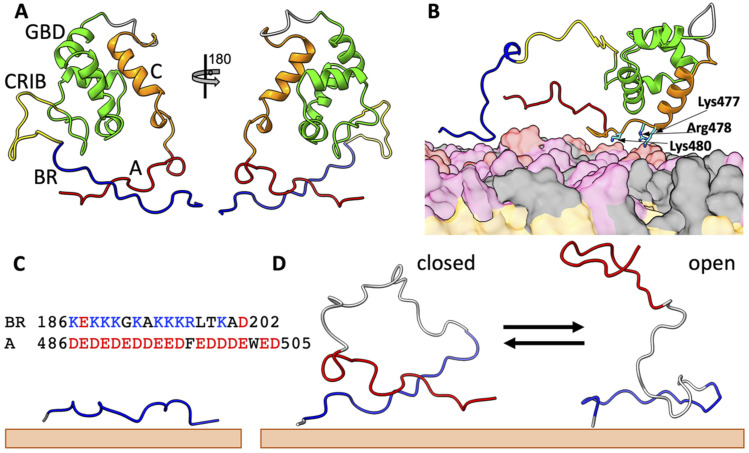
FIG. 1.
Structures of N-WASP in different states. (a) Autoinhibited state. The C-terminal central (“C”) motif forms a helix and is docked to the GTPase-binding domain (GBD), whereas the C-terminal acidic (“A”) motif locks with the basic region (BR) in a cross-arm pose. Note that, following the construct for the NMR structure 1EJ5,11 residues between the GBD (ending with Gln275) and the C motif (starting with Ala462) were replaced by a six-residue linker [sequence (GGS)2]. The N-terminal 185 residues (preceding the start of the BR at Lys186) were also not included. (b) An intermediate upon activation by PIP2. The A motif is released from the BR, but the C motif is still bound to the GBD, and three basic residues between the C-motif helix and the A motif bind to the membrane and keep the A motif in proximity to the BR. (c) A membrane-bound BR-only fragment. The sequences of the BR and the A motif are also shown. (d) A BR-A fragment undergoing closed-to-open transition on the membrane surface. BR, CRIB, GBD, C, and A are in blue, yellow, green, orange, and red, respectively; linkers are in gray. Lipids are shown as surface, with PIP2, PS, and PC headgroups in red, pink, and gray, respectively; lipid tails are in orange.