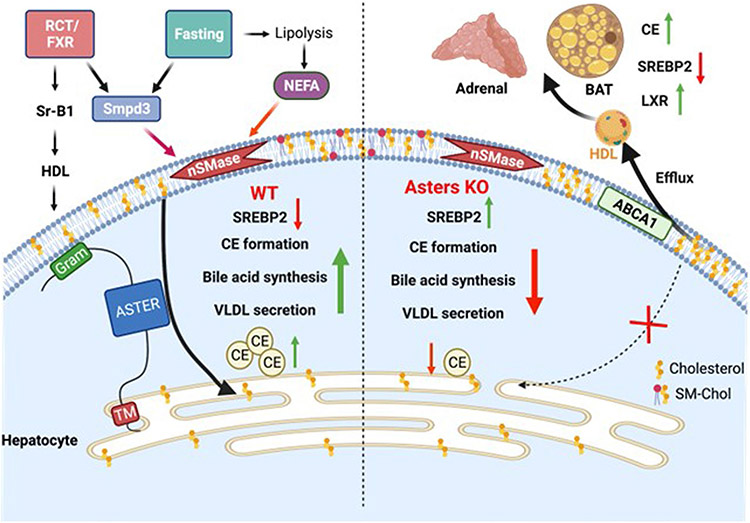
Extended Data Fig. 5 ∣. Model for the role of hepatic Asters in liver and systemic cholesterol homeostasis.
In normal physiology (left side of schematic), fasting stimulates fatty acid release from adipose tissue to promote hepatic sphingomyelinase activity, which liberates sequestered cholesterol in the hepatocyte PM. Aster proteins recognize this newly accessible cholesterol and transport it to the ER for CE formation, suppression of SREBP-2 pathway targets, bile acid synthesis and VLDL production. Hepatic Asters are also induced by FXR and function in the RCT pathway by moving HDL-derived cholesterol (and LDL-derived cholesterol) within hepatocytes. Loss of hepatic Aster function (right side of the schematic) impairs CE formation and VLDL output during fasting. Loss of Asters in the liver also decreases the appearance of HDL-derived cholesterol in bile and feces, raises plasma cholesterol levels (due to enhanced liver cholesterol efflux), and causes peripheral cholesterol accumulation (for example, adrenal gland, brown adipose tissue).