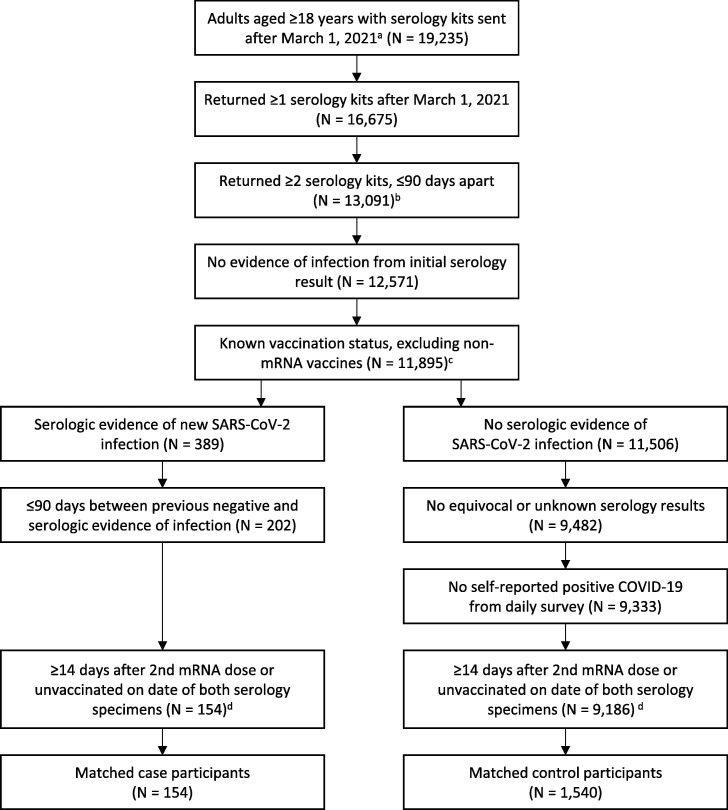
Fig. 1.
Participants of the COVID-19 Community Research Partnership Study included in analysis of vaccine effectiveness using serologic data. a. Among 29,187 adults enrolled in the study via one of the 4 included health systems in this analysis, 27,535 did not report a previous COVID-19 diagnosis at the time of study enrollment. Of these, 19,235 were sent at least one serology kit between March 1, 2021 and October 31, 2021, and 17,235 returned one or more serology dried blood spot specimens. b. Among 16,675 participants who returned ≥1 serology kits after March 1, 2021, 15,125 returned at least 2 serology kits. c. Among the 676 participants excluded, 625 were excluded because they only received one dose or a non-mRNA vaccine; the remaining 51 participants had an unknown vaccination status. d. Test kit results were excluded based on the timing of vaccination status if a participant was categorized as “partially vaccinated” or having received a third dose at the collection date for the previous negative test.