Abstract
Prognostic markers in advanced hepatocellular carcinoma (HCC) are relevant for clinical decisions. Variations in inflammatory indexes, such as neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR), may correlate with outcomes. In the present study, it was aimed to assess the prognostic role of inflammation indexes in patients with HCC and the evolutionary behavior of these variables within the first month of treatment in a cohort of patients treated with sorafenib from 2009-2021. Subgroups were divided based on the median of each variable (‘low’ or ‘high)’. Survival was estimated using the Kaplan-Meier method. Hazard Ratio (HR) with 95% confidence interval (CI) were estimated using Cox regression models. A total of 373 patients were included, most Child-Pugh-A (83.1%) and BCLC-C (74%). Child-Pugh-A (P=0.011), performance status 0 (P<0.001), no ascites (P<0.001) and NLR<2.6 (P<0.001) were independently associated with improved survival. Baseline PLR was not correlated with survival (P=0.137). Patients who maintained low NLR at baseline and at 1 month (reference subgroup) had improved survival (18.6 months, 95% CI:15.4-22.0) compared with the subgroup that maintained high NLR at baseline and at 1 month (4.2 months, 95% CI:3.6-5.9), with HR: 3.80 (95% CI: 2.89-4.96). The subgroup with low NLR at baseline and high NLR at 1 month had a worse prognosis compared with the reference group (HR:1.4, 95% CI: 1.1-2.0), whereas the subgroup with high NLR at baseline and low at 1 month had similar outcome (HR:1.2, 95% CI: 0.8-1.6). It was concluded that evolutionary variation of NLR has a prognostic role in HCC patients under systemic therapy. This finding suggested that systemic inflammation and early modulation of the immune environment during treatment may correlate with outcomes.
Keywords: liver cancer, systemic treatment, inflammation, prognosis, neutrophil-to-lymphocyte ratio
Introduction
Prognostic assessment is critical to guide therapeutic decision-making in hepatocellular carcinoma (HCC). Systemic treatment is the recommended approach for patients with advanced stage or intermediate stage that are not candidates for liver-directed modalities according to the Barcelona Clinic Liver Cancer (BCLC) staging system (1). Sorafenib was the first systemic agent approved on the basis of improved survival in two phase III trials (2,3). Lenvatinib has shown non-inferiority to sorafenib, while regorafenib, cabozantinib and ramucirumab provided survival benefit in the second-line setting after sorafenib (4-7). More recently, immunotherapy-based combinations such as atezolizumab plus bevacizumab and tremelimumab plus durvalumab were superior to sorafenib in phase III trials and are considered the standard first-line treatment for eligible patients (8,9).
As the treatment of HCC evolves, prognostic evaluation gains relevance for clinical counseling and risk stratification in clinical trials. Furthermore, assessing early benefit is challenging because traditional radiologic criteria may not capture the therapeutic activity of tyrosine-kinase inhibitors and immunotherapies in HCC (10-12).
Cancer-associated inflammation and the inflammatory response to tumor antigens are increasingly explored as determinants of clinical outcomes (13-15). Studies have shown that absolute immune cell count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), serum albumin and C-reactive protein play a prognostic role in several solid tumors, including HCC (16-20).
NLR and PLR are reproducible indexes, although there is no established cut-off and different values are used in published studies (16-20). These studies consider only baseline measurements, whereas evolutionary changes in these indexes over the course of treatment may refine their clinical utility. Thus, the longitudinal evaluation would provide the basis for interventions aimed at modulating the immune response, such as add-on or locoregional plus systemic combinations.
The objective of the present study was to investigate the prognostic impact of baseline and evolutionary variables related to inflammatory indexes after 1 month of treatment in a cohort of patients with HCC treated with sorafenib.
Materials and methods
Patients and methods
A database of 373 HCC patients consecutively treated at Instituto do Cancer do Estado de Sao Paulo (Brazil) from July 2009 to December 2021 was retrospectively evaluated. The population included consisted of patients with HCC diagnosed on the basis of radiologic and/or histologic criteria (21) who initiated first-line systemic treatment according to local policy. The study was approved by the local Ethics Committee and informed consent was waived due to the retrospective nature of the study (report 3.807.496).
Relevant data were collected from medical records including age, sex, performance status (PS) according to the Eastern Cooperative Oncology group (ECOG) scale, etiology, Child-Pugh score, BCLC stage, serum laboratory parameters at baseline and after 1 month (+/- 7 days) of treatment initiation, previous treatments for HCC, treatment duration and death. Data were last updated on 12th-December 2021.
NLR was defined as the peripheral blood absolute neutrophil count divided by the peripheral blood absolute lymphocyte count. PLR was defined as the peripheral blood absolute platelet count divided by the peripheral blood absolute lymphocyte count. Peripheral blood samples were collected at baseline (+/- 7 days from treatment initiation) and after 1 month (+/- 7 days). Patients were divided into groups according to the median values of the variables analyzed (high: ≥ than the median value; and low: < than the median value).
Treatment and assessments
During the study period, sorafenib was the first-line treatment available to patients who were candidates for systemic treatment. Sorafenib was administered orally at an initial dose of 400 mg twice daily, which could be modified upon development of adverse events according to type and severity. Clinical and laboratory assessments were performed at baseline and monthly, and radiology evaluation was performed bimonthly. Treatment was continued until symptomatic progression, radiological progression, treatment intolerance or death.
Statistical analysis
Quantitative variables were expressed as median and interquartile range (IQR; 25-75th percentiles). Categorical variables were described as absolute frequencies and percentages (%). Fisher's exact test was used to compare categorical variables. ANOVA was used to assess whether there is a relationship between the etiology of cirrhosis and inflammatory indexes. Time to event variables were described using the Kaplan-Meier method with median and their 95% confidence interval (CI). Survival functions were compared using the log-rank test.
Hazard ratios (HR) with their 95% CI were estimated using a Cox regression model (adjusted for the baseline variables with statistical significance in the univariate analysis). For the landmark analysis at 1 month of treatment, the cohort was classified into groups according to ‘baseline-1 month’ measurements into: high-high, low-low, high-low and low-high.
The prognostic accuracy of the models was evaluated by the Harrell's C concordance index, in which a higher C index indicates greater accuracy and agreement between predicted and expected outcomes. All tests were two-sided with a significant level of 0.05. Analysis was performed using Stata software version 15.1 (StataCorp LP).
Results
Baseline characteristics
A total of 373 patients were included. Baseline demographic and clinical characteristics are summarized in Table I. Median cohort age was 62.2 years (IQR: 55.1-68.9), most patients were male (n=282; 75.6%), with ECOG-PS 0 (n=196; 52.6%), Child-Pugh A class (n=310; 83.1%) and BCLC-C stage (n=276; 74%). A total of 200 (53.6%) patients received prior locoregional treatments for HCC. The median duration of sorafenib was 4.2 months (IQR; 2.3-7.9). A total of 61 (16.3%) patients underwent second-line systemic therapy after sorafenib discontinuation.
Table I.
Baseline characteristics of the cohort.
| Baseline characteristics | N=373 |
|---|---|
| Median age, (IQR) | 62.2 (55.1-68.9) |
| Sex | |
| Male, n (%) | 282 (75.6%) |
| Female, n (%) | 91 (24.4%) |
| Chronic liver disease etiology | |
| No hepatopathy, n (%) | 10 (2.7%) |
| Hepatitis C, n (%) | 153 (41%) |
| Hepatite B, n (%) | 50 (13.4%) |
| Alcohol, n (%) | 52 (13.9%) |
| NASH/NAFLD, n (%) | 35 (9,4%) |
| Others | 73 (19.6%) |
| Previous treatment* | |
| Transplant, n (%) | 21 (5.6%) |
| Resection, n (%) | 46 (12.3%) |
| Ablation, n (%) | 33 (8.9%) |
| TACE, n (%) | 164 (44%) |
| No previous treatment, n (%) | 173 (46.4%) |
| Child-Pugh class | |
| A, n (%) | 310 (83.1%) |
| B, n (%) | 63 (16.9%) |
| ALBI grade | |
| 1 | 137 (36.7%) |
| 2 | 209 (56.0%) |
| 3 | 27 (7.3%) |
| BCLC stage | |
| B, n (%) | 97 (26%) |
| C, n (%) | 276 (74%) |
| Macrovascular invasion, n (%) | 157 (42.1%) |
| Extrahepatic spread, n (%) | 155 (41.5%) |
| ECOG Performance status, n (%) | |
| 0-1, n (%) | 333 (89,2%) |
| 2, n (%) | 40 (19,8%) |
| Alpha-fetoprotein, median (IQR) | 209 (17-4.291) |
| Ascites, n (%) | 39 (10,5%) |
| Neutrophil-to-lymphocyte ratio, median (IQR) | 2.6 (1.9-4.0) |
| Platelet-to-lymphocyte ratio, median (IQR) | 106.7 (75-169.3) |
IQR, interquartile range; BCLC, Barcelona Clinic Liver Cancer Group; ECOG, Eastern Cooperative Oncology Group.
*Some patients have been treated with more than one previous treatment.
The median baseline neutrophil count was 3,455/mm3 (IQR: 2,300-4,650), the median baseline lymphocyte count was 1,200/mm3 (IQR: 900-1,725) and the median baseline platelet count was 141x103/mm3 (IQR: 84-214x103/mm3). The median baseline NLR was 2.6 (IQR: 1.9-4.0) and the median baseline PLR was 106.7 (IQR: 75-169.3).
Outcomes and baseline prognostic factors
At the last follow-up update, 333 (83.3%) patients had succumbed, 11 (2.9%) were lost to follow-up and 29 (7.7%) were alive. The median follow-up was 9.4 months (IQR: 4.3-19.1). The median overall survival (OS) of the entire cohort was 9.7 months (95% CI: 8.7-10.8 months). Considering the patients with Child-Pugh A and ECOG-PS 0 (n=182), the median OS was 19.6 months (95% CI: 15.7-21.9).
Univariate analysis was performed to identify potential baseline prognostic factors in the cohort. Child-Pugh class (A vs. B), ALBI grade (1 vs. 2/3), BCLC stage (B vs. C), vascular invasion (no vs. yes), ECOG-PS (0 vs. 1-2), ascites (no vs. yes), lymphocyte count (≥ vs. < median), NLR (< vs. ≥ median) and PLR (< vs. ≥ median) showed statistical significance and were evaluated in a multivariate model. On the other hand, baseline neutrophil and platelet counts were not statistically significant.
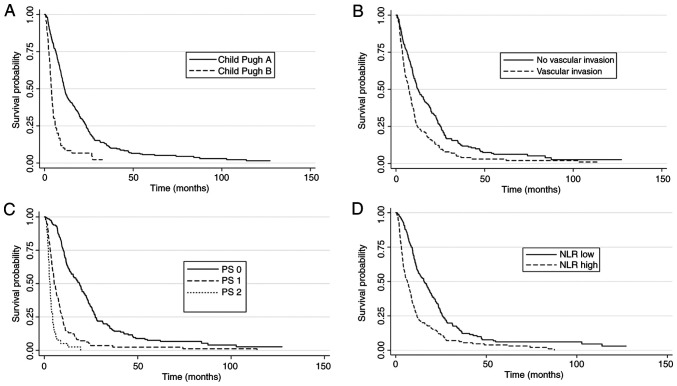
In the multivariate analysis, Child-Pugh A (P=0.011), ALBI grade 1 (P=0.005), absence of macrovascular invasion (P=0.03), ECOG-PS 0 (P<0.001), absence of ascites (P<0.001) and low NLR (P<0.001) were independently associated with improved OS (Table II and Fig. 1). Finally, there was no significant relationship between cirrhosis etiology and inflammatory indexes NLR (ANOVA P=0.07) and PLR (ANOVA P=0.06).
Table II.
Median overall survival, univariate and multivariate analysis according to subgroups.
| Subgroups | n | Median OS, months (95% CI) | Log rank (P-value) | Multivariate analysis HR (95% CI); P-value |
|---|---|---|---|---|
| Child-Pugh | <0.001 | 0.71 (0.59-0.84); 0.011 | ||
| A | 310 | 11.2 (10.2-12.8) | ||
| B | 63 | 3.8 (3.2-4.5) | ||
| ALBI grade | ||||
| 1 | 137 | 12.6 (10.7-18.3) | <0.001 | 1.40 (1.11-1.77); 0.005 |
| 2 | 209 | 8.8 (7.2-10.0) | ||
| 3 | 27 | 6.3 (3.3-10.1) | ||
| BCLC stage | 0.0015 | 1.02 (0.77-1.35); 0.219 | ||
| B | 97 | 14.4 (11.1-18.6) | ||
| C | 276 | 8.8 (7.5-10.1) | ||
| Vascular invasion | <0.001 | 1.31 (1.04-1.70); 0.030 | ||
| Yes | 157 | 7.2 (5.9-8.6) | ||
| No | 216 | 12.4 (10.6-15.7) | ||
| ECOG-PS | <0.001 | 0.52 (0.41-0.79); <0.001 | ||
| 0 | 196 | 18.8 (14.4-21.2) | ||
| 1-2 | 177 | 5.1 (4.6-6.4) | ||
| Neutrophils | 0.32 | |||
| Low | 186 | 10.8 (9.4-13.1) | ||
| High | 187 | 8.7 (7.1-10.1) | ||
| Lymphocytes | <0.001 | 1.22 (0.94-1.35); 0.128 | ||
| Low | 194 | 7.7 (5.3-8.8) | ||
| High | 179 | 11.9 (10.6-16.9) | ||
| Platelets | 0.334 | |||
| Low | 187 | 10.1 (8.5-11.2) | ||
| High | 186 | 9.4 (8.0-11.5) | ||
| Ascites | <0.001 | 0.74 (0.56-0.89); <0.001 | ||
| Low | 334 | 10.7 (9.7-11.9) | ||
| High | 39 | 3.5 (2.4-4.2) | ||
| NLR | <0.001 | 1.63 (1.27-2.10); <0.001 | ||
| High | 185 | 5.9 (4.4-7.4) | ||
| Low | 188 | 15.9 (12.6-18.9) | ||
| PLR | 0.002 | 1.27 (0.98-1.61); 0.137 | ||
| High | 185 | 7.6 (6.0-8.8) | ||
| Low | 188 | 12.1 (10.6-15.7) |
IQR, interquartile range; NASH, Non-alcoholic steatohepatitis; NAFLD, non-alcoholic fatty liver disease; TACE, transarterial chemoembolization; BCLC, Barceona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group.
Figure 1.
Survival curves according to prognostic factors. (A) Child-Pugh class A vs. B. (B) Vascular invasion no vs. yes. (C) PS 0 vs. 1-2. (D) NLR low vs. high. PS: Eastern performance status; NLR, neutrophil-to-lymphocyte ratio.
OS according to NLR evolution within 1 month of treatment
The landmark analysis at 1 month of treatment included 363 patients. A total of 10 (2.7%) patients were not included due to early treatment discontinuation as a result of symptomatic progression (n=4), limiting toxicity (n=2), patient decision to withdraw the treatment (n=1), lost to follow-up (n=1) and unavailable laboratory tests at 1 month (n=2). A total of 36 (9.7%) patients required dose reduction within the first month due to adverse events. The median interval between baseline and 1-month assessment was 32 days (IQR: 29-34 days).
Patients were classified into subgroups according to the ‘baseline-1 month’ NLR. Most patients were classified as low-low (n=124) or high-high (n=137). The subgroup with high-high NLR had worse survival (median OS: 4.2 months; 95% CI: 3.6-5.9) compared with the subgroup with low-low NLR (median OS: 18.6 months; 95% CI: 15.4-22.0) with a HR=3.8 (95% CI: 2.9-4.9). The subgroups with low-high and high-low NLR had intermediate median OS (11.1 months and 10.9 months, respectively). However, there was a statistical difference in OS between the low-low and low-high subgroups (HR, 1.4; 95% CI: 1.1-2.0; P=0.043), whereas there was no difference between the low-low and high-low subgroups (HR=1.2; 95% CI: 0.8-1.6; P=0.432). (Table III and Fig. 2).
Table III.
Survival according to NLR ratio at baseline and at 1 month of treatment.
| Subgroups (NLR baseline-1 month) | n | Median OS (95% CI) | HR (95% CI) |
|---|---|---|---|
| Low-low | 124 | 18.6 months (15.4-22.0) | 1 (reference) |
| Low-High | 46 | 11.1 months (6.3-13.4) | 1.40 (1,1-2.0) |
| High-low | 56 | 10.9 months (7.7-16.0) | 1.2 (0.8-1,6) |
| High-high | 137 | 4.2 months (3.6-5.9) | 3.8 (2.9-5.0) |
NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; CI, confidence interval; HR, hazard ratio.
Figure 2.
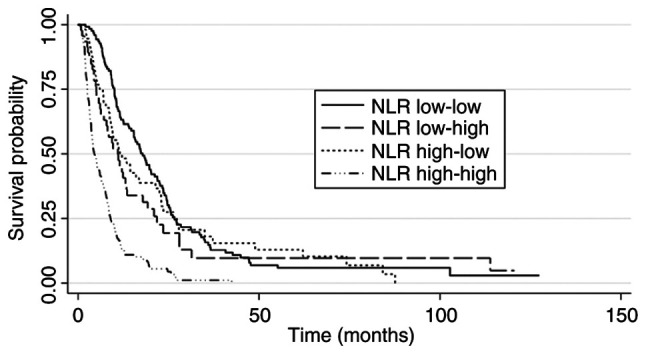
Survival curves according to subgroups based on NLR at baseline and 1-month. NLR, neutrophil-to-lymphocyte ratio.
Harrell's C indexes were calculated to assess the prognostic accuracy of the model containing NLR as a baseline parameter or as an evolutionary parameter measured at baseline and at 1 month. The model with evolutionary NLR showed a Harrell's C index=0.67 (95% CI: 0.64-0.70) which was superior to the prognostic ability of the NLR variable measured only at the baseline (Harrell's C index=0.62, 95% CI: 0.59-0.64), with a coefficient between the indexes of 0.05 (95% CI 0.03-0.06) and P<0.0001.
Although baseline PLR was not statistically significant in the multivariate analysis, the subgroups were also analyzed according to PLR evolution at baseline and at 1 month of treatment. Patients with low-low PLR (n=145) showed improved OS (14.1 months; 95% CI: 10.7-18.3) compared with patients with a high-high PLR (n=139) that presented a median OS of 6.5 months (95% CI: 5.0-8.4 months), with a HR=1.7 (95% CI:1.3-2.1). Similarly, the C-Harrell's index for the model with baseline PLR (0.57; 95% CI: 0.55-0.60) was inferior to the index for the model with evolutionary PLR (0.60; 95% CI: 0.57-0.63) with a coefficient between the indexes of 0.02 (95% CI: 0.01-0.03; P=0.001).
The radiological patterns of progression on sorafenib were reviewed in a subset of patients with evaluable imaging assessments (n=65). The onset of new extrahepatic lesions was associated with worse survival (median OS=8.1 months; 95% CI: 4.6-9.6) vs. other patterns of progression (median OS 13.1 months; 95% CI: 10-28.8) with a log rank P=0.01. However, there was no correlation between the pattern of progression and NLR evolution subgroups (P=0.078).
Integrating NLR and BCLC staging system
Patients were divided according to the NLR evolution at the 1st month across each BCLC stages (B and C) to evaluate the usefulness of this biomarker in defining prognostic subgroups within each of these stages.
It was observed that there is a statistically significant difference between the low-low and high-high NLR subgroups, both in patients classified in the BCLC-B stage and in the BCLC-C stage. Patients with low-high and high-low NLR had intermediate median OS. Patients who had high NLR at baseline and low at 1 month showed no difference in OS compared with patients with low NLR at baseline and 1 month in both BCLC stages (Table IV).
Table IV.
Survival according to NLR ratio at baseline and at 1 month of treatment and BCLC stages.
| NLR (baseline-1 month) | n | Median OS (95% CI) | HR (95% CI) | |
|---|---|---|---|---|
| BCLC-B stage | Low-low | 41 | 18.3 months (11.5-23.3) | 1 (reference) |
| Low-high | 17 | 9.4 months (3.9-21.8) | 1.37 (0.71-2.65) | |
| High-low | 15 | 21.4 months (11.2-48.9) | 0.84 (0.43-1.64) | |
| High-high | 23 | 8.4 months (4.7-12.6) | 2.21 (1.26-3.89) | |
| BCLC-C stage | Low-low | 83 | 17.4 months (12.5-22.5) | 1 (reference) |
| Low-high | 29 | 11 months (6.1-13.5) | 1.44 (0.91-2.27) | |
| High-low | 41 | 9.3 months (6.9-16.8) | 1.28 (0.86-1.89) | |
| High-high | 114 | 3.9 months (3.6-5.2) | 4.17 (3.03-5.73) |
BCLC, Barcelona Clinic Liver Cancer; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; CI, confidence interval; HR, hazard ratio.
Discussion
The present results identified that variations in systemic inflammation indexes, such as NLR and PLR, are early predictors of survival in patients with advanced HCC treated with sorafenib. Furthermore, the previously reported prognostic role of baseline NLR was validated (19). In addition, the incorporation of these biomarkers enhances the prognostic ability of the BCLC staging system to discriminate subgroups.
The natural history of advanced HCC is poor, but it improved significantly after the incorporation of tyrosine kinase inhibitors with antiangiogenic activity, such as sorafenib. These agents may also exert activity by facilitating T-cell recruitment, inhibiting T-reg cells and increasing T-lymphocyte trafficking into the tumor microenvironment (22-24). In recent years, immune checkpoint inhibitors have become the standard treatment after positive results in first-line trials, underscoring the concept that the immune system is a therapeutic target for achieving improved outcomes in HCC (25).
Cancer-associated systemic inflammation and the ability of cancer cells to evade immune destruction are considered key points of carcinogenesis (26). They encompass not only the direct interaction of immune and tumor cells, but also changes in hematopoiesis and lymphocyte activation in lymphoid organs, establishing a relationship between systemic and local inflammatory response (27-29). Circulating tumor cells clustered with neutrophils appear to present a particular transcriptome profile that determines a phenotype with more efficient metastasis formation (30). Besides, neutrophils may assume an immunosuppressive function in the tumor microenvironment through the expression of immunosuppressive cytokines (31,32). Lymphocytes, in turn, are recognized as having a pivotal role in antitumor immunity. Tumor-infiltrating lymphocytes are associated with improved outcomes in several tumor types, including HCC (33,34). When considered together, relative lymphopenia and neutrophilic leukocytosis may denote that the immune system is balanced in favor of a pro-tumor inflammatory response and the opposite behavior of neutropenia with lymphocytosis leads to an anti-tumor response pattern.
A meta-analysis of more than 40,000 patients reported that high baseline NLR is associated with worse prognosis across different types of solid tumors, including HCC (16). This finding was also reported by other groups that evaluated patients with HCC (17-20).
A relevant finding of the current study is that evolutionary measurements of NLR within the first month of systemic treatment is a predictor of OS. Since the correlation between radiologic response or time-to-progression with OS in HCC is faulty, this result suggests that an inflammatory switch reflected by the evolution of these markers may be a useful and accessible prognostic tool. The distinction between a higher likelihood of a favorable outcome (sustained low NLR at baseline and after 1 month of treatment) and a poor prognosis (high NLR at baseline and after 1 month) indicates the need for individualized follow-up strategies and a rationale for future studies testing early interventions in patients with worse predicted survival, such as add-on agents and combinations of immune-based therapies.
Notably, patients who had high baseline NLR and switched to low NLR at 1 month presented a survival outcome that tended to converge with the outcome of patients with low-low NLR. On the contrary, patients with low-high NLR diverged from the group with low-low NLR. Although the sample sizes of each subgroup are small, this observation suggests an early immune modulation associated with the treatment and/or the disease course. This assumption needs to be confirmed by translational data on the immunological processes that potentially drive these clinical observations.
Because neutrophils and platelets were not significantly associated with survival in the univariate analysis, it was hypothesized that there may be a more prominent role of lymphocytes counts in the indexes NLR and PLR. However, only NLR was independently associated with survival in the multivariate analysis, which suggests that NLR is more relevant than lymphocyte count individually or PLR. Besides, the highest concordance index was observed for the model containing NLR as an evolutionary parameter, suggesting that the evolution of NLR within the first month of treatment may carry a prognostic information. It is worth mentioning that the results of the present study require prospective and external validation.
A limitation to the current study is the retrospective nature, that prevents a precise identification of confounding factors, a distinction of immune cell populations represented in peripheral blood samples and their dynamics. Additional evaluations (for instance, at radiological progression) could emphasize the prognostic role of these inflammatory markers, but they were not widely available in this retrospective cohort. Furthermore, most patients started treatment in a period when sorafenib was the standard first-line treatment before the approval of immunotherapy combinations. Prospective data on the value of inflammatory indexes under immunotherapy are being collected by our group.
In conclusion, it was showed that the NLR has a clinically relevant prognostic role in HCC and the early evolution of this parameter identifies different outcomes. This finding is potentially associated with a background immune modulation during systemic treatment. Tumor burden, liver function and performance status are the cornerstones of prognostic assessment in HCC. However, additional prognostic factors can support the rationale for exploring a risk-based allocation into separate treatment strategies and optimizing decisions at a time when the HCC algorithms gain complexity.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LGDF and FJC developed the original idea for the study. LGDF, LFU, GFS and PSDA collected the data. LGDF, LFU, GFS and PSDA performed the statistical analysis and prepared figures and tables. LGDF, LFU, GFS, PSDA, RSDSMA, ALC, VAFA an FJC analyzed and interpreted data. LGDF, LFU, GFS and PSDA were responsible for writing the manuscript. LGDF, LFU, GFS, PSDA, RSDSMA, ALC, VAFA an FJC revised the manuscript critically for important intellectual content. LGDF, LFU and GFS confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The present study was approved (approval no. 3.807.496) by the Research Ethics Committee of University of Sao Paulo School of Medicine (Brazil).
Patient consent for publication
was waived by the local Research Ethics Committee (Comite de Etica para Analise de Projetos de Pesquisa CAPPesq-Hospital das Clinicas da Faculdade de Medicina da Universidade de São Paulo-HCFMUSP).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni E. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2100070. for the HIMALAYA Investigators. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 10.Rimola J, Da Fonseca LG, Sapena V, Perelló C, Guerrero A, Simó MT, Pons M, De La Torre-Aláez M, Márquez L, Calleja JL, et al. Radiological response to nivolumab in patients with hepatocellular carcinoma: A multicenter analysis of real-life practice. Eur J Radiol. 2021;135(109484) doi: 10.1016/j.ejrad.2020.109484. [DOI] [PubMed] [Google Scholar]
- 11.Kolamunnage-Dona R, Berhane S, Potts H, Williams EH, Tanner J, Janowitz T, Hoare M, Johnson P. Sorafenib is associated with a reduced rate of tumour growth and liver function deterioration in HCV-induced hepatocellular carcinoma. J Hepatol. 2021;75:879–887. doi: 10.1016/j.jhep.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019;16:617–630. doi: 10.1038/s41575-019-0179-x. [DOI] [PubMed] [Google Scholar]
- 13.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vesely MD, Schreiber RD. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 16.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(dju124) doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 17.Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, Himmelsbach V, Schulze K, von Felden J, Fründt TW, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy-development and validation of the CRAFITY score. J Hepatol. 2022;76:353–363. doi: 10.1016/j.jhep.2021.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Luen SJ, Savas P, Fox SB, Salgado R, Loi S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology. 2017;49:141–155. doi: 10.1016/j.pathol.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 19.da Fonseca LG, Barroso-Sousa R, Bento Ada S, Blanco BP, Valente GL, Pfiffer TE, Hoff PM, Sabbaga J. Pre-treatment neutrophil-to-lymphocyte ratio affects survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Med Oncol. 2014;31(264) doi: 10.1007/s12032-014-0264-5. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 21.EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. [DOI] [PubMed] [Google Scholar]
- 22.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated neutrophils recruit macrophages and T-Regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Romero AI, Chaput N, Poirier-Colame V, Rusakiewicz S, Jacquelot N, Chaba K, Mortier E, Jacques Y, Caillat-Zucman S, Flament C, et al. Regulation of CD4(+)NKG2D(+) Th1 cells in patients with metastatic melanoma treated with sorafenib: Role of IL-15Rα and NKG2D triggering. Cancer Res. 2014;74:68–80. doi: 10.1158/0008-5472.CAN-13-1186. [DOI] [PubMed] [Google Scholar]
- 24.Mathew NR, Baumgartner F, Braun L, O'Sullivan D, Thomas S, Waterhouse M, Müller TA, Hanke K, Taromi S, Apostolova P, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24:282–291. doi: 10.1038/nm.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7(7) doi: 10.1038/s41572-021-00245-6. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 30.Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 31.Wu WC, Sun HW, Chen HT, Liang J, Yu XJ, Wu C, Wang Z, Zheng L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci USA. 2014;111:4221–4226. doi: 10.1073/pnas.1320753111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 33.Martin D, Rödel F, Winkelmann R, Balermpas P, Rödel C, Fokas E. Peripheral leukocytosis is inversely correlated with intratumoral CD8+ T-Cell infiltration and associated with worse outcome after chemoradiotherapy in anal cancer. Front Immunol. 2017;8(1225) doi: 10.3389/fimmu.2017.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, Darcy PK, Loi S. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13(202) doi: 10.1186/s12916-015-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or and analyzed during the current study are available from the corresponding author on reasonable request.