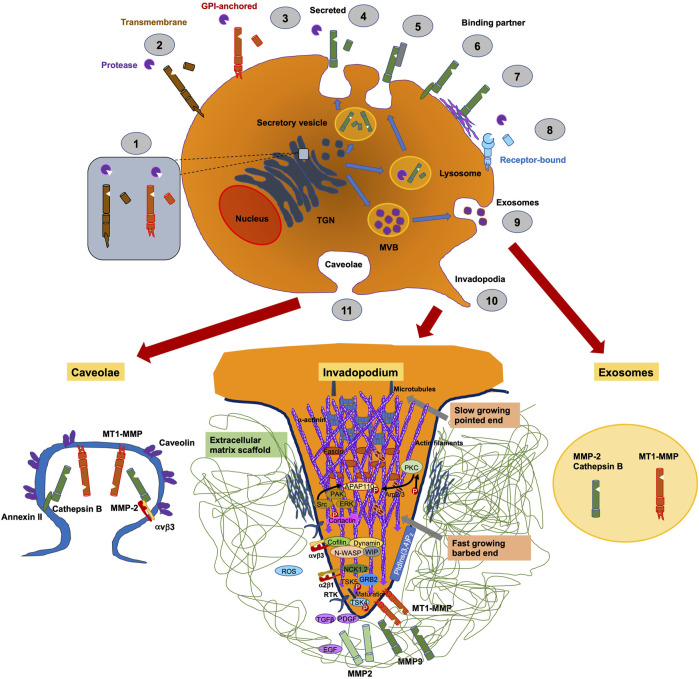
FIGURE 2.
Pericellular proteases, such as ADAM8, are involved in trafficking of proteins. They are synthesized in the ER and get transferred to the Golgi complex until they enter the trans-Golgi-network (TGN). (1) In the TGN membrane, attached proteases are switched active through furin. (2) Membrane-bound proteases are delivered to the cell surface, whereby they are inactive precursor proteins and become active within the perivascular cavity. (3) Traditionally, secreted proteases are transported to the cell membrane via a constitutive secretory pathway and activated after their liberation in the pericellular cavity. Proteases in endosomes or lysosomes are transported to the extracellular cavity by different pathways: (4) the secretory pathway, where they are secreted as proenzymes that must be activated by proteolysis, or (5) the mechanism of lysosomal exocytosis, where they are activated and thereafter secreted. Secreted proteases can bind to the cell surface through interference with other molecules, including CD44, integrins or (6) annexin II or through (7) tethering to ECM compounds. (8) Specific secreted proteases are attached to distinct receptors, for example, uPA couples to uPAR. (9) Proteases are capable to be liberated through exosomes originating from multivesicular bodies (MVBs), where they can be released into the extracellular cavity or into neighboring cells. The aggregation or the regional liberation of pericellular proteases are linked to microdomains on cell membranes, such as invadopodia, which are actin-rich moieties (10) or caveolae, which belong to “lipid raft” domains (11).