Abstract
Bone marrow is the primary site of blood cell production in adults and serves as the source of osteoblasts and osteoclasts that maintain bone homeostasis. The medullary microenvironment is also involved in malignancy, providing a fertile soil for the growth of blood cancers or solid tumors metastasizing to bone. The cellular composition of the bone marrow is highly complex, consisting of hematopoietic stem and progenitor cells, maturing blood cells, skeletal stem cells, osteoblasts, mesenchymal stromal cells, adipocytes, endothelial cells, lymphatic endothelial cells, perivascular cells, and nerve cells. Intercellular communication at different levels is essential to ensure proper skeletal and hematopoietic tissue function, but it is altered when malignant cells colonize the bone marrow niche. While communication often involves soluble factors such as cytokines, chemokines, and growth factors, as well as their respective cell-surface receptors, cells can also communicate by exchanging metabolic information. In this review, we discuss the importance of metabolic crosstalk between different cells in the bone marrow microenvironment, particularly concerning the malignant setting.
Keywords: Bone marrow, Cell metabolism, Cellular communication, Stromal cells, Leukemia, Bone metastasis
Highlights
-
•
The bone marrow microenvironment undergoes extensive remodeling in malignancy.
-
•
Broad metabolic exchange takes place between bone marrow stromal and cancer cells.
-
•
Metabolic interactions support cancer cell growth and therapy resistance.
-
•
Cancer cells often suppress osteogenesis and induce lipolysis in marrow adipocytes.
-
•
Targeting metabolic crosstalk has therapeutic potential in leukemia and bone metastasis.
1. Introduction
As a consequence of the violent movements of tectonic plates 1.5 billion years ago, tremendous amounts of minerals, such as calcium carbonate, were released into the oceans. This allowed oceanic organisms to develop an exoskeleton, which was later converted into an endoskeleton as the next major evolutive trait, eventually giving rise to vertebrates (Wagner and Aspenberg, 2011). The development of an endoskeleton allowed animals to expand their activity radius and colonize entirely new environments. During the transition from an aquatic to a terrestrial environment, increased exposure to ultraviolet light exerted a selective evolutionary pressure on several tissues, including the hematopoietic system. Consequently, while hematopoietic stem and progenitor cells are found in the kidney marrow in teleost fish, these cells reside in the bone marrow in terrestrial vertebrates where they are protected from ultraviolet light by the cortical bone (Kapp et al., 2018). Over 200 million years of co-evolution of blood and bone have led to the creation of a complex bone marrow ecosystem that orchestrates the production of both hematopoietic and skeletal cells. In modern day mammals, the bone marrow is composed of many different cell types, extracellular matrix, secreted factors such as cytokines, chemokines and growth factors, and metabolic cues (Morrison and Scadden, 2014). Functionally, it serves as the primary site for hematopoiesis, is involved in immune responses, and represents an important source of osteoblast and osteoclast precursors that are needed to maintain skeletal health. The bone marrow is also the preferred site for several cancers, including hematological malignancies such as leukemias and multiple myeloma, and metastasis of breast and prostate cancer.
2. The cellular diversity of the bone marrow microenvironment
The mammalian bone marrow is a complex organ. In the long bones of the appendicular skeleton, it is composed of a medullary canal surrounded by cortical bone and of smaller compartments located in between bone trabeculae found at the long bone ends. In most bones of the axial skeleton there is no central marrow canal, and bone marrow is found dispersed throughout the cancellous bone located between the cortices of smaller flat and short bones. The bone marrow is highly vascularized, with large arteries in the central marrow space branching into small arterioles that end in capillaries near the metaphyseal and endosteal regions (Fig. 1). These transition zone capillary vessels connect to the dense, fenestrated, and highly branched sinusoidal network of the marrow cavity that drains into the central veins carrying blood out of the marrow (Hendriks and Ramasamy, 2020). In addition to this extensive blood vessel network, recent evidence shows that the bone and bone marrow also contain lymphatic vessels (Biswas et al., 2023). Most insights into the cellular composition of the bone marrow have come from mouse studies, where engineered mouse strains have allowed researchers to identify and localize different cell types in the bone marrow and determine their functional role in hematopoiesis or bone formation. However, the human hematopoietic niche appears to be similar in most aspects (van Pel et al., 2016). In the following paragraphs, we aim to provide a concise overview of the different bone marrow microenvironments from both the hematopoietic and skeletal point-of-view. For more in-depth reading on hematopoietic and skeletal microenvironments in the bone marrow, we refer the reader to several excellent recent review papers (Ambrosi and Chan, 2021; Comazzetto et al., 2021; Mendez-Ferrer et al., 2020; Pinho and Frenette, 2019; Sanchez-Lanzas et al., 2022).
Fig. 1.
The cellular composition of the bone marrow niche. Several stromal cell types are involved in the maintenance of HSCs and the support of hematopoiesis in the bone marrow. In the central bone marrow niche, LepR+ adipo-CAR cells and sinusoidal endothelial cells provide CXCL12 and SCF to support HSC retention and function, while megakaryocytes secrete CXCL4 and TGF-β1, preserving HSC quiescence. Myelopoiesis also takes place in the central bone marrow, in niches distinct from those where HSCs are found. NG2+ periarteriolar cells and non-myelinating Schwann cells secrete respectively CXCL12 and TGF-β, involved in maintaining quiescent HSCs near arterioles in the endosteal and central bone marrow regions. In the endosteal niche, Osteolectin+LepR+ osteo-CAR cells provide CXCL12 and SCF to lymphoid-biased HSCs and early lymphoid progenitors. Osteoprogenitors further support B cell maturation, while mature osteoblasts maintain early T cell progenitors. The role of mature adipocytes in the bone marrow is complex and incompletely understood, but they are thought to support HSCs by providing fatty acids.
Since mature blood cells are predominantly short-lived, maintenance of hematopoiesis throughout life relies on the presence of hematopoietic stem cells (HSCs). HSCs can generate all functional blood cells (multipotency) and give rise to new HSCs during cell division (self-renewal). HSCs reside in a specialized bone marrow microenvironment known as the HSC niche, representing the functional and anatomical unit where signals from the periphery are integrated to govern appropriate HSC behavior during steady-state and in response to stress. Anatomically, two main HSC niches with distinct cellular composition are considered: the endosteal niche, defined by its proximity to the bone surface – which can be cortical or trabecular – and the central bone marrow niche, which comprises >90 % of the bone marrow volume (Morrison and Scadden, 2014; Mendez-Ferrer et al., 2020).
The endosteal niche is characterized by the presence of osteogenic cells, including osteoprogenitors and osteoblasts, and arteriolar as well as transition zone capillary blood vessels. The endosteal niche was initially thought to be the only bone marrow HSC niche, a concept based on classic in vitro experiments showing that osteoblastic cells are an important source of hematopoietic cytokines and support the maintenance of HSCs in culture (Benayahu et al., 1992; Taichman and Emerson, 1994), and on early studies with genetically-altered mice in which osteoblast pool expansion correlated with increased numbers of primitive hematopoietic cells (Calvi et al., 2003; Zhang et al., 2003). However, it is now appreciated that both the endosteal and central bone marrow niches harbor primitive HSCs, with likely both overlapping and distinct functions for both niches (Ambrosi and Chan, 2021; Comazzetto et al., 2021; Mendez-Ferrer et al., 2020; Pinho and Frenette, 2019; Sanchez-Lanzas et al., 2022). In addition, more advanced mouse experiments have revealed that the endosteal niche may be of particular importance for lymphopoiesis, with Osterix (Osx)+ osteoprogenitors having a prominent role in B cell maturation, while Osteocalcin-expressing mature osteoblasts are required to maintain early T cell progenitors and for T cell specification (Ding and Morrison, 2013; Yu et al., 2015; Zhu et al., 2007).
The central bone marrow niche is characterized by the abundance of sinusoidal blood vessels covered by leptin receptor (LepR)-expressing perivascular mesenchymal stromal cells (MSCs), as well as arterioles with mesenchymal neural-glial antigen 2 (NG2)+ cells that in mice are also identifiable by their high expression of a Nestin-GFP transgene. HSC visualization through histology shows that under homeostatic conditions, the vast majority (>85 %) of primitive HSCs are closely associated with the sinusoids (Acar et al., 2015). However, this association may simply result from the high abundance of sinusoids in the central marrow (Kokkaliaris et al., 2020). The endothelial cells and LepR+ stromal cells are both essential components of the sinusoidal niche, providing key factors that promote HSC maintenance such as C-X-C motif chemokine ligand 12 (CXCL12) and stem cell factor (SCF) (Ding and Morrison, 2013; Ding et al., 2012a). Direct contact between HSCs and sinusoidal blood vessels is required, as HSCs have been shown to depend mainly on the membrane-bound form of SCF (Barker, 1994). Another essential cell type of the sinusoidal niche is megakaryocytes, which produce transforming growth factor (TGF)-β1 and CXCL4 to limit HSC proliferation (Bruns et al., 2014; Zhao et al., 2014). Recent evidence shows that the sinusoidal niche is also the site of myelopoiesis, and interestingly revealed that granulocyte and monocyte/dendritic cell differentiation occurs in different sinusoidal sub-niches, which are distinct from the sinusoidal HSC niche (Zhang et al., 2021).
The role of arterioles and their periarteriolar NG2+ cells, enriched in the endosteal niche but also found in the central bone marrow, remains an area of active research and debate (Comazzetto et al., 2021; Pinho and Frenette, 2019). While only a small percentage of HSCs are located next to arterioles and periarteriolar NG2+ cells are not an important source of SCF, these cells may control HSC quiescence through secreted factors such as CXCL12 (Asada et al., 2017; Kunisaki et al., 2013). Periarteriolar niches appear to be particularly important for lymphoid-biased HSCs, while myeloid-biased HSCs prefer the sinusoidal niche (Pinho et al., 2018). The periarteriolar niche further supports HSC quiescence by maintaining HSCs in a low reactive oxygen species (ROS) state (Itkin et al., 2016). Interestingly, arterioles are also intimately associated with sympathetic nerve fibers (Kunisaki et al., 2013; Mendez-Ferrer et al., 2010) and non-myelinating Schwann cells that maintain HSC dormancy through TGF-β secretion (Yamazaki et al., 2011). Circadian noradrenaline secretion from sympathetic nerves controls CXCL12 expression by periarteriolar cells, resulting in the rhythmic release of HSCs to the periphery (Mendez-Ferrer et al., 2010; Mendez-Ferrer et al., 2008).
The LepR+ perivascular cells not only provide growth factors for the maintenance of HSCs; they are also the major source of bone and adipocytes in adult bone marrow (Zhou et al., 2014). LepR+ cells are heterogeneous, including skeletal stem cells (SSCs) and osteogenic and adipogenic progenitors (Baryawno et al., 2019; Tikhonova et al., 2019). Recent findings from the Morrison group show that expression of an osteogenic growth factor, Osteolectin, distinguishes periarteriolar LepR+ cells poised to undergo osteogenesis from perisinusoidal LepR+ cells poised to undergo adipogenesis (Shen et al., 2021). The hormone leptin, produced by adipocytes, can directly act on LepR+ MSCs and promotes their adipogenic differentiation at the expense of osteogenesis (Yue et al., 2016). Leptin also controls bone mass by acting on LepR-expressing hypothalamic neurons activating the sympathetic nervous system, which inhibits osteogenesis via β2-adrenergic receptors present on osteoblasts (Takeda et al., 2002).
When malignant cells colonize the bone marrow, they often seem to hijack HSC niches and can induce extensive niche remodeling favoring disease progression at the expense of normal hematopoiesis (Mendez-Ferrer et al., 2020; F. Chen et al., 2021; Kokkaliaris and Scadden, 2020; Witkowski et al., 2020). Subsets of cancer cells with a quiescent phenotype, such as leukemic stem cells or dormant breast metastatic cells, are frequently found in perivascular regions, where they are maintained by endothelial cells and MSCs secreting CXCL12 and TGF-β (Agarwal et al., 2019; Ghajar et al., 2013; Nobre et al., 2021; Saito et al., 2010; Vinado et al., 2022). Actively proliferating cancer cells progressively change the niche to an extent where normal hematopoiesis is severely impacted. MSCs and cells of the osteoblastic lineage are often impacted in the malignant setting, although the phenotype can differ depending on the type of cancer growing in the bone marrow, as detailed below. How bone marrow niche cells can support malignant cell dormancy, growth and therapy-resistance is still being studied. While many efforts have focused on cytokines, growth factors and adhesion molecules, it is becoming increasingly clear that metabolic interactions between stromal and malignant cells may play a key role in disease establishment and progression. Here, we provide an overview of the current knowledge on metabolic crosstalk in the malignant bone marrow niche.
3. Metabolic interactions between stromal and blood cancer cells
3.1. Myeloid malignancies
Myeloid malignancies are bone marrow disorders occurring when HSCs and their progenitor cells of the myeloid differentiation branch display abnormal proliferation, self-renewal, and differentiation (Duncavage, 2022). As a result, functional blood cell production is perturbed, and the bone marrow often becomes hypercellular. Myeloid malignancies comprise myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPN), chronic myeloid leukemia (CML), and acute myeloid leukemia (AML) (Arber et al., 2022). MDS are characterized by the overproduction of immature progenitor cells, associated with reduced or abnormal mature blood cells. In MPN in contrast, there is an overproduction of mature blood cells such as erythrocytes, platelets or myeloid cells that gradually accumulate in the bone marrow and peripheral blood. CML, often classified under MPN, is linked to the presence of the BCR::ABL1 translocation and characterized by increased levels of immature hematopoietic stem or myeloid progenitor cells, mostly without overt changes in mature blood cells (Arber et al., 2022). MDS, MPN and CML progress relatively slow, but they display pre-leukemic characteristics and have the propensity to progress into AML. AML is characterized by a rapid clonal expansion of immature myeloid progenitor cells in the bone marrow, blood, and other hematopoietic organs such as the spleen and liver. AML is a very heterogeneous disease, with inter- and intra-clonal genetic heterogeneity existing between and within patients (Ding et al., 2012b; Miles et al., 2020; Morita et al., 2020). There is also cellular heterogeneity in AML, with cellular hierarchies composed of leukemia-initiating cells, also called leukemic stem cells (LSCs), forming myeloid blasts and cells with mature phenotypes (van Galen et al., 2019). The heterogeneity between AML clones or between patients is also reflected at the epigenomic, transcriptomic, and metabolic level (Erdem et al., 2022a; Klco et al., 2014; Li et al., 2020), creating substantial challenges for the treatment of this dismal disease as the persistence of residual cells after therapy is very common.
Growth of malignant myeloid cells in the bone marrow leads to substantial changes in niche cell populations (Ghobrial et al., 2018). Progressive AML induces remodeling of the endosteal niche with loss of endosteal vessels, sympathetic nerves, periarteriolar cells and mature osteoblasts, and expansion of the osteoprogenitor pool (Baryawno et al., 2019; Duarte et al., 2018; Hanoun et al., 2014). MPN also cause neuroglial damage, leading to apoptosis of periarteriolar Nestin-GFP+ MSCs (Arranz et al., 2014). In myelofibrosis, a subtype of MPN, increased cytokine production leads to fibrosis originating from LepR+ perivascular cells (Decker et al., 2017). The presence of CML cells in contrast is associated with overproduction of dysfunctional hyperinflammatory osteoblasts that poorly support normal HSCs, while MSC numbers remain stable (Schepers et al., 2013). In MDS, increased MSC apoptosis and production of inflammatory cytokines have been described (Flores-Figueroa et al., 2002; Medyouf et al., 2014).
Alterations in the cellular composition of the bone marrow niche also impact its nutritional characteristics, which can benefit malignant myeloid cells that have different metabolic dependencies compared to their healthy counterparts. For example, while the bone marrow is already considered to have low oxygen tensions in health (Spencer et al., 2014), the growth of AML cells in the bone marrow leads to vascular leakiness and increases hypoxia, creating an environment that allows AML growth at the expense of normal hematopoiesis and that protects AML cells from chemotherapy-induced cell death (Passaro et al., 2017). The cellular effects of hypoxia are often mediated by hypoxia-inducible factor 1 alpha (HIF1α) signaling, and in accordance, high expression of HIF1α was detected in 67 % of AML patients (Deeb et al., 2011) and 49 % of MDS patients, where it correlates with poor prognosis (Tong et al., 2012). Intriguingly, while loss or inhibition of HIF1α has adverse effects on disease progression in CML (H. Zhang et al., 2012), JAK2V617F-positive MPN (Baumeister et al., 2020) and MDS (Hayashi et al., 2018), the impact in AML is less clear, with studies showing both pro-tumorigenic (Wang et al., 2011) or tumor-suppressive effects of HIF1α (Velasco-Hernandez et al., 2014). Differences between humans and mice, oncogenic drivers or disease stage may underlie this discrepancy, and further research will be needed to unravel the role(s) of HIF1α in AML.
One of the major downstream effects of HIF signaling is a reprogramming of cellular metabolism, with one key feature being the activation of glycolysis (Fig. 2). Glycolysis has been shown to support leukemogenesis in CML and AML (Wang et al., 2014), although at least in AML, glycolytic metabolism does not seem to be under the direct control of HIF signaling (Wierenga et al., 2019). Molecular imaging studies using a genetically encoded metabolic sensor for the cytoplasmic NADH/NAD+ ratio (SoNar) revealed that AML LSCs with a more glycolytic profile home to the hypoxic endosteal bone marrow niche (Hao et al., 2019). This process is regulated by the enzyme pyruvate dehydrogenase kinase 2 (PDK2), which limits pyruvate entry into the mitochondrial tricarboxylic acid (TCA) cycle. Pyruvate can then be converted into lactate, a reaction that regenerates NAD+ from NADH, allowing cells to maintain a high glycolytic flux which requires high cytoplasmic NAD+ availability. Interestingly, the endosteal niche has also been shown to have much higher ATP levels than the vascular niche, which acts as a homing signal for AML LSCs (He et al., 2021). By acting on P2X7, ATP enhances calcium flux-mediated phosphorylation of CREB, inducing phosphoglycerate dehydrogenase (PHGDH) expression to maintain serine metabolism and LSC fate. The presence of AML cells further increases bone marrow ATP levels. While the source of ATP remains unknown, the differential ATP levels in the endosteal versus vascular niche suggest that cells unique to this microenvironment, such as osteoblasts or osteoclasts, may be responsible. Collectively, these studies suggest that the metabolic preference of malignant myeloid cells can determine their physical localization in the bone marrow, which they can further alter to create a self-reinforcing pro-tumorigenic niche.
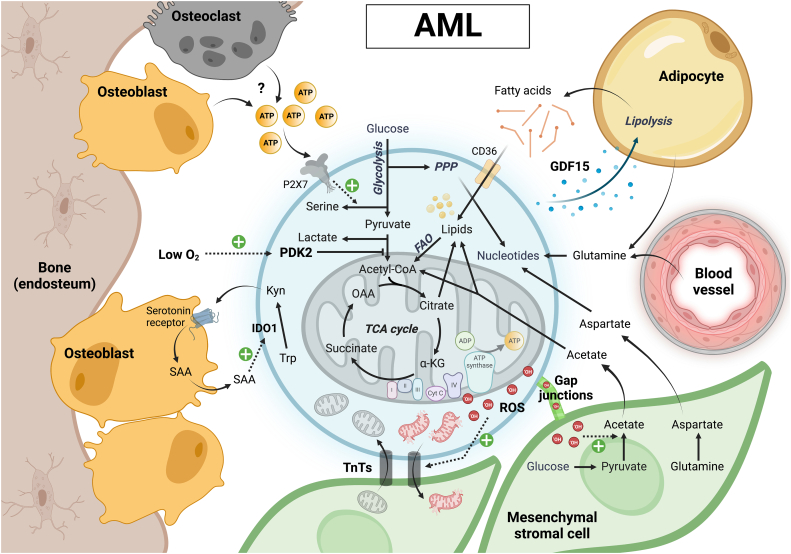
Fig. 2.
Metabolic crosstalk between AML cells and bone marrow stromal cells. AML LSCs with a more glycolytic profile home to the hypoxic endosteal bone marrow niche, where high environmental ATP –from a yet unknown cellular source– additionally promotes leukemic serine biosynthesis. Metabolic crosstalk in the endosteal niche also includes a vicious cycle of AML-derived kynurenine and osteoblast-derived acute-phase protein serum amyloid A (SAA) production, leading to niche remodeling in favor of cancer progression. AML LSCs and blasts with a more oxidative metabolism may preferentially localize close to and interact with adipocytes or MSCs. Release of GDF15 by AML cells triggers adipocyte lipolysis, providing fatty acids for oxidation in the AML cells. Transfer of ROS from AML cells to MSCs stimulates acetate production, which is also taken up by AML cells to fuel their TCA cycle and support lipid synthesis. AML mitochondria damaged by excessive ROS can be transferred to MSCs via TnTs, and the cancer cells receive healthy mitochondria via a similar mechanism in return. MSCs also provide glutamine-derived aspartate to AML cells, allowing the latter to synthetize nucleotides necessary for proliferation and DNA damage repair.
In addition to their glycolytic metabolic profile, AML blasts and LSCs also display an active mitochondrial metabolism and depend on oxidative phosphorylation (OXPHOS) for their survival (Erdem et al., 2022b; Lagadinou et al., 2013; Molina et al., 2018). However, while AML cells have more mitochondria and increased respiration compared to their normal counterparts, they exhibit an abnormal OXPHOS process characterized by low spare respiratory capacity and poor ATP generation, presenting therapeutic possibilities (Nelson et al., 2021; Sriskanthadevan et al., 2015). Despite this abnormal mitochondrial metabolism, most AML LSCs are characterized by relatively low levels of ROS (Lagadinou et al., 2013), indicating that they can efficiently dispose of ROS or limit ROS production. The mitochondrial TCA cycle in AML LSCs seems to be fueled predominantly by fatty acids (German et al., 2016; Samudio et al., 2010; Tcheng et al., 2021), and fatty acid oxidation (FAO) further increases in the setting of relapse after chemotherapy (Farge et al., 2017) or during treatment with the BCL2 (B-cell lymphoma 2) inhibitor venetoclax (Stevens et al., 2020). At least part of the lipids used by AML cells to fuel FAO is provided by adipocytes in the bone marrow microenvironment, which are induced by leukemia cells to undergo remodeling and lipolysis (Shafat et al., 2017; Tabe et al., 2017). Adipocyte remodeling appears to be triggered by GDF-15 secreted from AML cells (Lu et al., 2018), and renders the bone marrow niche less supportive of normal myelo-erythropoiesis (Boyd et al., 2017). A similar metabolic crosstalk may occur in CML, where LSCs have been shown to utilize gonadal adipose tissue as a niche that allows them to evade chemotherapy by inducing adipocyte lipolysis to fuel FAO in a subpopulation of LSCs expressing the fatty acid transporter CD36 (Ye et al., 2016).
Several recent studies have identified additional stroma-derived metabolites required for AML cell growth and post-therapy survival. Vilaplana-Lopera and colleagues found that AML cells prompt bone marrow MSCs to secrete acetate to fuel their TCA cycle and lipid biosynthesis (Vilaplana-Lopera et al., 2022). The presence of AML cells increases glycolysis in the stromal cells, and the transfer of ROS from AML to stromal cells via gap junctions triggers the conversion of pyruvate to acetate. In our own previous research, we showed that residual AML cells persisting after chemotherapy exhibit transient metabolic adaptations enabling their survival in the bone marrow. To support their synthesis of pyrimidine nucleotides, the residual AML cells took up large amounts of glutamine and required niche-derived aspartate (van Gastel et al., 2020). The origin of the imported glutamine is most likely the circulation, although adipocytes may also be a source of glutamine in the bone marrow (Ehsanipour et al., 2013).
Metabolic signaling in the opposite direction also exists in AML. For example, AML cells produce excessive amounts of kynurenine, which acts as an oncometabolite that remodels the endosteal niche to the cancers advantage (Galan-Diez et al., 2022). In a positive feedback mechanism, the secreted kynurenine induces a proinflammatory state through serotonin receptor signaling in osteoblasts, leading to secretion of acute-phase protein serum amyloid A that upregulates expression of IDO1 – the rate-limiting enzyme for kynurenine production – in AML cells, thus favoring cancer progression (Galan-Diez et al., 2022).
Besides uni- and bidirectional metabolite exchange, organelle exchange is observed between niche cells and leukemic blasts. MSCs have been shown to actively transfer functioning mitochondria via tunneling nanotubes (TnTs) to AML cells, supporting leukemia cell growth and resistance to chemotherapy (Moschoi et al., 2016; Saito et al., 2021). TnTs are F-actin-based membrane tubes, sometimes also containing microtubules, that form between cells and allow intercellular communication ranging from electrical signaling to the transfer of organelles (Dupont et al., 2018). The generation of ROS in AML cells by the enzyme NADPH oxidase 2 (NOX2) or as a consequence of mitochondrial dysfunction is an important trigger for this transfer. The mitochondrial transfer may support AML cell bioenergetics and antioxidant defenses, as suggested by a study from the Méndez-Ferrer group investigating the ability of Nestin+ MSCs to protect AML LSCs from chemotherapy (Forte et al., 2020).
In summary, it is now well-established that myeloid malignancies are linked to extensive remodeling of the bone marrow niche, providing increased metabolic support of cancer cell growth and therapy evasion.
3.2. Lymphoid leukemias
Lymphoblastic leukemia originates from genetic lesions occurring in blood progenitor cells that are committed to differentiating into either the T cell (15 %) or B cell (85 %) lineage, including mutations that impart the capacity for unlimited self-renewal or that induce stage-specific developmental arrest, leading to the accumulation of immature blasts in the bone marrow, peripheral blood and often also in the central nervous system (CNS) (Malard and Mohty, 2020). Unlike AML, acute lymphoblastic leukemia (ALL) incidence is common in pediatric populations and has a good prognosis. Pediatric ALL has a 5-year overall survival rate of 90 %, whereas the 5-year overall survival rate of adult ALL is only 40 %. Drug resistance is the primary cause of disease recurrence and relapse, which might be due to alterations in cell-intrinsic (Park et al., 2011) and cell-extrinsic (Bradstock et al., 1996; Macanas-Pirard et al., 2012) anti-apoptotic pathways allowing a small population of cells to persist. Reported literature suggests that in ALL the leukemia-initiating cells are widely nonspecific; even the more differentiated cells give rise to leukemia in the xenograft model, unlike the hierarchical model reported in AML (McClellan and Majeti, 2013; Cox et al., 2004; Castor et al., 2005).
While metabolic reprogramming has been less extensively studied in ALL compared to other blood cancers, several studies indicate that both B-ALL and T-ALL cells exhibit hyperactivation of OXPHOS, supporting leukemogenesis and therapy resistance (C. Chen et al., 2021; Kishton et al., 2016; Matthijssens et al., 2021). Similar to AML LSCs (Lagadinou et al., 2013), leukemia-initiating cells in T-ALL are characterized by low levels of ROS despite increased OXPHOS activity (Giambra et al., 2012). While leukemic T cells can utilize aerobic glycolysis to support proliferation, this pathway is surprisingly less active in T-ALL cells compared to proliferating normal T cells (Kishton et al., 2016). Whether this is linked to the different OXPHOS rate or due to other metabolic demands in leukemic versus normal T cells has yet to be investigated. The metabolic profile of ALL cells is also impacted by their microenvironment, suggesting a remarkable plasticity. ALL cells are found in very different locations, including the bone marrow, spleen, and CNS, each of which is substantially different in nutritional composition. In the bone marrow, cytokines released by ALL cells induce adipocytes to activate lipolysis and provide free fatty acids to ALL cells, shifting the leukemia cells from glucose oxidation to FAO (Tucci et al., 2021). In contrast, within the CNS niche, ALL cells are found in the cerebrospinal fluid which is low in oxygen and several nutrients including fatty acids, creating a particular dependence for these cells on de novo lipid synthesis (Savino et al., 2020).
Like myeloid malignancies, ALL cells induce extensive niche remodeling when growing in the bone marrow. In B-ALL, leukemia cells disrupt the normal perivascular niches, reducing CXCL12 production at these sites (Colmone et al., 2008). Instead, the malignant cells produce large amounts of SCF, attracting healthy HSCs but inhibiting their correct functioning. B-ALL cells also secrete CCL3 and TGF-β1, recruiting Nestin+ MSCs from sinusoidal niches and promoting their transition into cancer-associated fibroblast-like cells that help form chemoprotective islands (Duan et al., 2014). T-ALL cells in the bone marrow do not seem to occupy or depend on any specific niche, but induce rapid, selective remodeling of the endosteal space (Hawkins et al., 2016). As a result, perivascular MSCs are maintained but mature osteoblastic cells are completely lost, severely compromising normal hematopoiesis. ALL cells also secrete extracellular vesicles (EVs), consisting of lipid bilayers that act as carriers for amino acids, proteins, RNA, miRNA, and DNA. EVs derived from B-ALL cells have been shown to induce a metabolic switch from OXPHOS to aerobic glycolysis in nearby stromal cells (Fig. 3). These stromal cells release excess lactate in the microenvironment, which can be used by the tumor cells as a source of energy, a phenomenon often referred to as the reverse Warburg effect (Johnson et al., 2016). Besides affecting the bone marrow niche, EVs from ALL patients were also shown to compromise the hematopoietic potential of healthy HSCs (Georgievski et al., 2022).
Fig. 3.
Metabolic interactions in the ALL bone marrow niche. The ALL bone marrow is characterized by an accumulation of MSCs at the expense of osteoblasts. MSCs convert glucose to lactate, which fuels the mitochondrial metabolism of ALL cells in addition to fatty acids provided by adipocytes. High ROS levels promote the exchange of damaged versus healthy mitochondria between ALL cells and MSCs via TnTs. MSCs also help ALL cells to maintain their redox balance by converting cystine to cysteine, which after transfer to the leukemic cells supports reduced glutathione (GSH) synthesis. MSCs further protect ALL cells from amino acid-depleting therapies such as L-Asparaginase or PEG-Arginase by respectively providing asparagine or arginine on which the ALL cells depend for their survival and growth.
It is well recognized that many adhesive interactions between ALL cells and MSCs contribute to the self-renewal and survival of leukemic cells, such as lymphocyte function-associated antigen (LFA-1)/intercellular adhesion molecule-1 (ICAM-1)-mediated and very late antigen-4 (VLA-4)/vascular cell adhesion molecule-1 (VCAM-1)-mediated adhesion (Jacamo et al., 2014; Peled et al., 2018). The other form of intercellular connections recently recognized is the formation of TnTs, where the cell extends filopodia-like protrusions toward the adjacent or neighboring cells, as described in AML. ALL cells form TnTs with stromal cells in the BM to transfer and exchange mitochondria, autophagosomes, and ICAM-1 (de Rooij et al., 2017). In B-ALL, DNA-damaging chemotherapeutic agents such as daunorubicin and cytarabine promote activation of the stromal cells via ROS and result in the formation of TnTs, transferring mitochondria to the leukemic cells as a chemoprotective mechanism (Burt et al., 2019). Inhibition of TnT formation by microtubule inhibitors such as vincristine sensitizes leukemia cells to chemotherapy. In T-ALL, leukemia cells transfer their damaged mitochondria to stromal cells and maintain a low number of functional mitochondria as a mechanism to reduce intracellular ROS levels (Wang et al., 2018). Neutralization of ICAM-1 significantly reduced TnT formation and restored the sensitivity of T-ALL cells to chemotherapy (Wang et al., 2018).
Another way in which stromal cells can help lymphoid leukemia cells to maintain low ROS levels is by modulating cysteine availability. Cysteine, typically present in its oxidized form cystine in the extracellular space, is regarded as the rate-limiting substrate for the synthesis of the antioxidant glutathione. In an elegant study, Zhang and colleagues demonstrated that primary chronic lymphoid leukemia (CLL) cells exhibit a limited capacity to take up cystine due to low expression of the xc− transporter (W. Zhang et al., 2012). Bone marrow MSCs in contrast import cystine, convert it to cysteine and release it back into the extracellular space where it is taken up by CLL cells, allowing the cancer cells to synthesize glutathione and protecting them from chemotherapy-induced cytotoxicity.
Interactions between MSCs and lymphoid leukemia cells appear to play a pivotal role in the resistance to metabolic therapies. L-asparaginase is one of the frontline therapies in the management of ALL (Egler et al., 2016). Due to their low expression of asparagine synthetase, lymphoblasts are auxotrophic for the non-essential amino acid asparagine. L-asparaginase depletes the extracellular pool of asparagine, thereby promoting leukemic cell death (Egler et al., 2016). However, it has been reported that stromal cells protect ALL cells from L-asparaginase-mediated cell death by providing asparagine (Chiu et al., 2021; Iwamoto et al., 2007). ALL blasts synthesize and secrete glutamine, which is taken up by MSCs and used to produce asparagine. In accordance, MSCs from ALL patients have increased expression of SLC38A5, an exporter of asparagine to the extracellular milieu (Chiu et al., 2021). Like asparagine, arginine is an essential amino acid for some ALL cells. Treatment with pegylated arginase, depleting extracellular arginine levels, is toxic to T-ALL cells in culture and reduces disease burden and extends lifespan in mouse B-ALL xenografts (De Santo et al., 2018; Kwong-Lam and Chi-Fung, 2013). Similar to the effects of L-asparaginase, the presence of MSCs rescues arginase-induced toxicity, although the underlying mechanism is still unclear (De Santo et al., 2018; Kwong-Lam and Chi-Fung, 2013).
We anticipate that in the coming years, further understanding of the metabolic interactions occurring in lymphoblastic leukemias growing in the bone marrow and other microenvironments will provide the basis for novel therapeutic strategies.
3.3. Multiple myeloma
Multiple myeloma (MM) is a blood cancer of monoclonal plasma cells that accumulate in the bone marrow (van de Donk et al., 2021). It is the second most common hematological malignancy in high-income countries. MM typically starts as an asymptomatic precursor condition such as monoclonal gammopathy of undetermined significance (MGUS) or smoldering MM, in which the initiating genetic abnormalities are already present. Progression to overt MM is complicated by multi-organ dysfunction, including hypercalcemia, renal insufficiency, anemia, and bone destruction (van de Donk et al., 2021). While the introduction of new therapies has significantly extended survival, most MM patients will die of their disease due to eventual relapse and therapy resistance development (van de Donk et al., 2021).
The bone marrow microenvironment plays a key role in MM progression (Ghobrial et al., 2018). Most genetic mutations appear to be acquired very early in disease development, at the asymptomatic precursor stage (Bolli et al., 2018), and transition to malignancy may be facilitated by changes in the microenvironment. Bone marrow MSCs have been recognized as critical regulators of myeloma cell adhesion, migration, proliferation, and survival through direct cell-cell contact or by secreting cytokines such as interleukin-6 (IL6), CXCL12 and insulin-like growth factor 1 (IGF1) (Ghobrial et al., 2018). The MSCs also appear to be irreversibly changed, with reduced osteogenic differentiation potential and increased expression of inflammatory cytokines (Reagan and Ghobrial, 2012). In addition, EVs secreted by MSCs derived from MM patients promote cancer cell growth, while those obtained from healthy donor MSCs are tumor suppressive (Roccaro et al., 2013).
Bone destruction, one of the main features of advanced MM, is associated with enhanced osteoclast activity stimulated by the secretion of IL6 and RANK ligand (RANKL) by MM cells, and with reduced osteoblast differentiation and activity. This creates a disequilibrium that favors bone loss, creating a feedback loop driving further MM cell proliferation and immune suppression because of the release of IGF1 and TGF-β during bone resorption (Terpos et al., 2018). Secretion of osteoblast-inhibiting factors such as DKK1 and sclerostin is one way by which MM cells inhibit osteoblast activity (Terpos et al., 2018), but metabolic signals are also involved (Fig. 4). Some MM cells express high levels of thymidine phosphorylase, which converts thymidine into thymine and 2-deoxy-d-ribose-1-phosphate that is further dephosphorylated into 2-deoxy-d-ribose (2DDR) (Liu et al., 2016). MM cell-secreted 2DDR binds to integrins αVβ3/α5β1 in osteoblast progenitors, activates PI3K/Akt signaling, increases DNMT3A expression and methylation of RUNX2 and Osx, leading to decreased osteoblastogenesis (Liu et al., 2016). The secreted 2DDR also binds to integrin αVβ3 in osteoclast progenitors, activates PI3K/Akt signaling, and increases DNMT3A expression and methylation of IRF8, leading to increased NFATc1 expression and osteoclastogenesis (Liu et al., 2016). Finally, 2DDR acts on osteocytes, where it increases expression of MHC class II transactivator (CIITA) through STAT1/IRF1 signaling (Liu et al., 2022). CIITA induces RANKL and sclerostin production by osteocytes, which respectively promote osteoclast and inhibit osteoblast differentiation, thus promoting bone destruction.
Fig. 4.
Short- and long-distance metabolite exchanges in MM. Avid glutamine consumption by MM cells leads to osteoblast depletion and bone destruction. Secretion of 2-deoxy-d-ribose (2DDR) by MM cells further contributes to this phenotype. Low microenvironmental glutamine levels trigger glutamine synthesis in MSCs, but this process does not appear to be sufficient in osteoblasts, which need large amounts of glutamine to differentiate and function. Urea, released in large amounts due to the hyperactive nitrogen metabolism of MM cells, stimulates the growth of nitrogen-recycling bacteria in the gut that through glutamine synthesis promote MM progression. Besides glutamine catabolism, MM cells exhibit high rates of glycolysis, a phenotype that is reinforced by bone marrow hypoxia. Low oxygen levels in the bone marrow also promote production of the immune-suppressive metabolite adenosine by the cancer cells. MM cells additionally take up lactate produced by MSCs and induce fatty acid release by adipocytes, although it is still unclear whether these metabolites fuel oxidative phosphorylation or have a different metabolic fate in the MM cells. As observed for other blood cancer cells, MM cells exhibit mitochondrial exchange with MSCs, trading their damaged for functional mitochondria.
MM cells also change the metabolic composition of the bone marrow. Both MM cell lines and patient-derived cells take up large amounts of glutamine to fuel their TCA cycle (Bolzoni et al., 2016). Accordingly, glutamine levels in the bone marrow plasma of MM patients are depleted, and glutamine synthetase is activated in stromal cells (Bolzoni et al., 2016). Since glutamine is a key nutrient for osteoblast differentiation, proliferation, and bone formation (Chiu et al., 2020; Stegen et al., 2021; Yu et al., 2019), the loss of bone mass in MM may be additionally linked to glutamine depletion in the bone marrow microenvironment.
Besides glutamine catabolism, MM cells rely heavily on glycolysis, even though they can engage mitochondrial OXPHOS when needed (Bloedjes et al., 2021). The glycolytic phenotype of MM cells appears to be driven by genetic alterations as well as the low oxygen levels of the bone marrow microenvironment (Bloedjes et al., 2021). Like in other lymphoid malignancies, MM cells can take up lactate from their microenvironment, where it is released by MSCs, other stromal cells or the cancer cells themselves (Fujiwara et al., 2015). The fate of this lactate in MM cells remains however unclear. With MM progression, the bone marrow becomes hypoxic (Hu et al., 2012), which may favor the creation of an immune-suppressive microenvironment in which the cancer cells increase adenosine production (Boison and Yegutkin, 2019). Indeed, bone marrow plasma from MM patients shows increased levels of adenosine as well as CD39 and CD73, the enzymes responsible for converting ATP to adenosine (Yang et al., 2020). Adenosine blocks T cell activity and, in accordance, inhibition of both CD39 and CD73 increases the immune response and abrogates tumor progression in a mouse model of MM (Yang et al., 2020).
Another stromal cell type altered in the setting of MM are bone marrow adipocytes, which may not be surprising given the link between obesity and risk of MM progression (Fairfield et al., 2021; Panaroni et al., 2022). The number of adipogenic stromal cells, pre-adipocytes and adipocytes is increased in the bone marrow of premalignant MGUS and smoldering MM patients, but not in overt MM (Panaroni et al., 2022). In culture, premalignant MM cells stimulate adipogenic differentiation of stromal cells, and adipocytes promote premalignant MM cell proliferation (Panaroni et al., 2022). In overt MM, bone marrow lipolysis is seen, and fatty acids released in this process are taken up by MM cells and fuel their proliferation (Fairfield et al., 2021; Panaroni et al., 2022). Malignant MM cells also alter adipocyte gene expression and cytokine secretory profiles, leading to ROS generation, mitochondrial damage, and induction of a senescent-like phenotype in adipocytes (Fairfield et al., 2021). The altered bone marrow adipocytes in turn protect MM cells from treatment-induced cell-cycle arrest and apoptosis (Fairfield et al., 2021), and may contribute to MM-induced bone lesions that do not heal even when the disease is in remission (Liu et al., 2019), making them an interesting target for therapeutic intervention (Fairfield et al., 2021).
As observed for other blood cancers, mitochondrial transfer from bone marrow MSCs to cancer cells has been observed in MM. Mitochondrial transfer in MM is bi-directional, occurring via TnT formation and partial cell fusion (Marlein et al., 2019; Matula et al., 2021). The transfer is strongly increased upon chemotherapy treatment, increasing ATP levels and lowering ROS levels in the MM cells, while increasing stromal ROS (Matula et al., 2021). Thus, mitochondrial activity, even if usually low in MM, can be important for these cells. In that regard, low OXPHOS activity can be a marker for venetoclax sensitivity in MM, and drugs inhibiting mitochondrial electron transport chain subunits sensitize MM to venetoclax treatment in mouse models (Bajpai et al., 2020).
Emerging fields of biomedicine, such as those exploring the importance of the microbiome, can lead to interesting discoveries regarding heterocellular metabolic crosstalk. In a recent study, Jian and colleagues uncovered a vicious cycle where MM patients presented with a dysregulated gut microbiome composition, which in turn favored MM progression (Jian et al., 2020). The accumulation of urea nitrogen in MM stimulates the growth of nitrogen-recycling bacteria such as Klebsiella and Streptococcus in the gut, which promote MM progression via de novo synthesis of glutamine. Other metabolites produced by gut bacteria, such as short-chain fatty acids, are also known to impact bone homeostasis (Zaiss et al., 2019). We anticipate that in the coming years, continuing research in this area will significantly increase our understanding of the importance of these short-chain fatty acids and other bacterial metabolites for bone health and pathology, including for the growth of cancer cells in the bone marrow microenvironment.
4. Metabolic interactions between stroma and metastatic solid tumor cells
Metastasis is a life-threatening outcome in patients with cancer. It is a complex process involving the acquisition of invasive properties and tumor cell interactions with different microenvironments. Bone is the most frequent site of metastasis of two of the most common cancers, breast cancer and prostate cancer (Zhang et al., 2020). At the early stages of metastasis, the acquired migratory and invasive properties are associated with epithelial-mesenchymal transition (EMT), encompassing changes in cell morphology and interactions with neighboring cells and the extracellular matrix (Devignes et al., 2018). Recent studies have shown that the extravasation of breast cancer cells from the primary tumor site is improved by hypoxia exposure, as it induces the expression of cell adhesion molecules that enable the interaction with endothelial cells (Valiente et al., 2014). Similarly, key EMT inducers such as TWIST (twist basic helix-loop-helix transcription factor 1), ZEB1 (zinc finger E-box binding homeobox 1) or SNAI1 (snail family transcriptional repressor 1) are directly activated by HIF signaling in several cancer models (Yang et al., 2008). In patients with bone metastasis, there are two types of lesions: osteolytic lesions, primarily found in breast cancer, and osteoblastic lesions, which are common in prostate cancer. Mixed lesions can also occur, especially at the early stages of bone colonization (Roodman, 2004). The bone lesions are characterized by an imbalance between the formation of new bone by osteoblasts and the resorption of mineralized bone by osteoclasts, leading to clinical complications such as pathological fractures, bone pain and metabolic disturbances, which severely reduce the quality of life (Chen et al., 2010).
4.1. Breast cancer metastasis
Breast cancers are traditionally divided into three subtypes: estrogen receptor-positive (ER+; often further subdivided by hormone receptor status and proliferative characteristics into low-risk luminal A and high-risk luminal B subtypes), human epidermal growth factor receptor 2 positive (HER2+), and triple negative breast cancer (TNBC; where cells lack the expression of ER, HER2 and progesterone receptor). All three subtypes metastasize most frequently to bone compared to other organs, although the incidence of bone metastasis is slightly higher in the HER2+ subtype compared to the others (Wu et al., 2017). Most bone lesions associated with breast cancer metastasis are osteolytic, linked to enhanced osteoclastogenesis and osteoclast activation by metastatic breast cancer cells, leading to overall bone loss (Chen et al., 2010). However, breast cancer cells have also been shown to inhibit osteogenic differentiation by secreting the Wnt antagonist sclerostin (Mendoza-Villanueva et al., 2011). In accordance, sclerostin inhibition reduces metastases and prevents breast cancer-induced bone destruction by promoting osteoblast-mediated bone formation (Hesse et al., 2019).
Hypoxia can contribute to establishing the premetastatic niches in different tissues through the secretion of systemic signals, such as lysyl oxidase (LOX), by the hypoxic tumor cells (Erler et al., 2009). The main function of LOX is to induce collagen crosslinking and matrix stiffness. In bone, LOX induces IL6 expression and interacts with RANKL, resulting in an acceleration of the bone resorption process by osteoclasts. LOX can be secreted from primary tumors into the circulation, leading to its accumulation in distant organs such as bone where it primes the tissue for colonization by inducing the formation of premetastatic lesions (Devignes et al., 2018; Clézardin et al., 2021). Once breast cancer cell colonization is established, the local LOX production increases, which further increases IL6 and RANKL levels, osteoclast activity and bone loss (Devignes et al., 2018). Similar to the vicious circle of bone loss observed in MM, the release of growth factors such as IGF1 and TGF-β from the bone matrix during osteoclastic resorption stimulates tumor cell growth and osteolytic factor production, favoring bone resorption adjacent to the tumor cells (Chen et al., 2010). In addition, the presence of the cancer cells induces osteoblast senescence, associated with increased IL6 production that further stimulates osteoclastogenesis (Luo et al., 2016).
The different breast cancer subtypes have not only different proliferation and metastasis capabilities, but also distinct metabolic traits (Fig. 5). ER+ tumors show an equilibrium between OXPHOS and glycolysis, sometimes also exhibiting a reverse Warburg effect through uptake of lactate released by cancer-associated fibroblasts, while most TNBC cells show a classical Warburg metabolism characterized by high aerobic glycolysis and lactic acid fermentation, and low OXPHOS (Elia et al., 2016). HER2+ tumors display higher expression of glutamine and lipid metabolism-related proteins compared to other subtypes (Kim et al., 2013; Kim et al., 2015). Nevertheless, metabolic heterogeneity may also exist within breast tumors (Kondo et al., 2021), and the interplay of cancer cells with their microenvironment further influences metabolic profiles (Dias et al., 2019). In this view, it is interesting to note that breast cancer cells metastasizing to different organs display extensive metabolic heterogeneity and engage distinct metabolic programs depending on their site of metastasis (Dupuy et al., 2015). A recent study further showed that TNBC metastatic tumors adopt a metabolic signature resembling that of primary tumors of their destination tissue (Roshanzamir et al., 2022). The extent of adaptation, however, varies across different organs, and the metastatic tumors also retain metabolic features resembling their TNBC origin.
Fig. 5.
Subtype-dependent metabolic crosstalk in bone-metastatic breast cancer. Most metastatic breast cancer cells in the bone microenvironment exhibit an active mitochondrial metabolism fueled by glucose, glutamine, MSC-derived lactate, and adipocyte-derived fatty acids. Mitochondria transferred from endothelial cells and MSCs further boost the oxidative capacity of the cancer cells. Glutamine metabolism in metastatic breast cancer cells also supports the biosynthesis of serine, a metabolite that is not only essential for cancer cell survival in the metastatic niche, but that additionally promotes osteoclastogenesis and bone destruction. TNBC cells induce bone loss through a different mechanism. These cells exhibit high rates of glycolysis and fermentation, releasing high amounts of lactate that promotes osteoclast activity and bone resorption. In turn, secretion of PUFAs by osteoclasts promotes proliferation, migration, and survival of breast cancer cells. While the osteoblast-osteoclast balance is disturbed in the bone marrow invaded by breast cancer cells, osteoblasts are still present and secrete several metabolites that favor tumor growth, including LPA, PGE2 and deoxycholate. Osteocytes can have pro- or anti-tumorigenic effects on breast cancer cells depending on their relative secretion of ATP and adenosine. This ratio can change in response to oxidative stress, and ectonucleotidase expression by the cancer cells can further shift the ratio between these two metabolites in favor of tumor cell growth and migration.
In a pioneering study, Dupuy and colleagues discovered that breast cancer cells with broad metastatic potential use both OXPHOS and glycolysis, while cells with site-selective metastatic potential engage OXPHOS (bone- or lung-metastatic cells) or glycolysis-dependent (liver-metastatic cells) metabolic strategies (Dupuy et al., 2015). They further showed that the mitochondrial metabolism of bone- and lung-metastatic breast cancer cells is fueled by both glucose and glutamine oxidation (Dupuy et al., 2015). Comparison of bone-tropic breast cancer cells with parental cell lines further revealed that bone-metastatic cells have increased dependency on extracellular glutamine for their survival (Tandon et al., 2021). This dependency is linked with serine biosynthesis, allowing the cells to resist low glucose levels in the metastatic niche (Tandon et al., 2021). Similar results were found by Pollari et al., who linked this feature to increased osteoclastogenesis as well (Pollari et al., 2011). Serine proved to be an essential nutrient for osteoclasts (Pollari et al., 2011), and excessive serine biosynthesis by breast cancer cells occupying the bone marrow niche could therefore be one factor contributing to metastasis-related bone loss. Highly glycolytic MDA-MB-231 cells, a TNBC cell line, also induce osteolytic lesions, but appear to do so via a different mechanism. The aggressive TNBC cells secrete large amounts of lactate, which is taken up by osteoclasts through monocarboxylate transporter 1 (MCT1) (Lemma et al., 2017). In accordance, inhibition of MCT1 abrogates bone resorption in mice with metastatic breast cancer (Lemma et al., 2017).
Reverse metabolic communication from osteoclasts to breast cancer cells also occurs, and seems to involve several lipid molecules. The presence of breast cancer cells increases osteoclast secretion of polyunsaturated fatty acids such as arachidonic acid and eicosapentaenoic acid, both prometastatic lipids, and decreases secretion of lysophosphatidylcholines, which are antimetastatic (Krzeszinski et al., 2017). The mechanisms through which these lipids influence cancer cell proliferation, migration and survival are still unknown.
Lipids in the breast cancer microenvironment are also provided by adipocytes, which are induced by tumor cells to activate lipolysis and release fatty acids into their surroundings, fueling the cancer cell's metabolism. Breast cancer cells preferably colonize the adipose niche in the bone marrow, where they are in close contact with adipocytes (Soni et al., 2021). Exposure to lipids supplied by marrow adipocytes induces expression of the fatty acid transporter CD36, the lipid chaperone fatty acid-binding protein 4 (FABP4), and pro-inflammatory IL1β in metastatic breast cancer cells and stimulates their growth and invasiveness (Herroon et al., 2013). In addition to fatty acids, bone marrow adipocytes are also a source of adipokines such as leptin and adiponectin, and of inflammatory cytokines, all of which promote tumor progression (Soni et al., 2021). Given this nefarious role of adipocytes, it is not surprising that obesity doubles the chance to develop breast cancer and promotes its progression and metastasis (Evangelista et al., 2019).
Another player in this intercellular lipid crosstalk is osteoblasts. Release of the bile acid salt sodium deoxycholate by osteoblasts promotes cell survival and induces the migration of metastatic breast cancer cells (Silva et al., 2006). Osteoblasts also produce bioactive lipids including lysophosphatidic acid (LPA) and prostaglandin E2 (PGE2) (Saier et al., 2021). LPA acts on osteoclasts by promoting survival and cytoskeleton rearrangement, thus favoring bone resorption, and stimulates proliferation, survival, and migration of bone metastatic cancer cells (Saier et al., 2021). LPA also stimulates inflammatory cytokine production by both osteoblasts and tumor cells. In addition to LPA, breast cancer cells increase the production of PGE2 by osteoblasts, promoting bone metastasis through different mechanisms (Ohshiba et al., 2003). PGE2 can directly enhance tumor cell growth, survival, migration, and invasion (Saier et al., 2021), but also induces recruitment of regulatory T cells to bone metastatic sites thus allowing escape from immune surveillance (Karavitis et al., 2012). Breast cancer cells also produce PGE2, which increases very early in the metastatic process and is required for the cancer cells to colonize the bone microenvironment (Saier et al., 2021; Singh et al., 2007). The effects of PGE2 on bone cells are complex, as this molecule can stimulate both bone formation and resorption (Saier et al., 2021). PGE2 can act directly on osteoblasts and stimulate their differentiation (Saier et al., 2021). However, PGE2 also increases RANKL expression by osteoblasts and has been shown to stimulate the differentiation of mouse osteoclast precursors into osteoclasts. In combination with the fact that it stimulates pro-osteoclastic cytokine production by tumor cells, PGE2 is generally considered to favor bone destruction in breast cancer metastasis (Saier et al., 2021), although further research is warranted.
In contrast to osteoblasts, which play a mostly tumor-supportive role in breast cancer metastasis, bone-embedded osteocytes were shown to reduce tumor cell growth and migration. Osteocytes release ATP through connexin 43 hemichannels, and ATP binds to the purinergic P2X7 receptor in breast cancer cells and inhibits their migration and growth (Zhou et al., 2015; Zhou et al., 2016). To escape this effect, breast cancer cells can convert ATP to adenosine through the action of the CD39 and CD73 ectonucleotidases (Petruk et al., 2021). Opposite to ATP, adenosine promotes breast cancer cell migration and growth (Zhou et al., 2015), and may have additional inhibitory effects on immune cells as observed in MM (Yang et al., 2020), although this remains to be confirmed in the metastatic breast cancer setting. Elevated oxidative stress in the tumor-occupied bone marrow microenvironment can also increase adenosine secretion by osteocytes (Tian et al., 2021), changing the ATP-to-adenosine ratio in favor of tumor cell growth.
Like the other types of cancer growing in bone, breast cancer cells communicate with stromal cells in a contact-dependent manner through gap junctions and TnTs. Transfer of microRNAs from bone marrow stromal cells to breast cancer cells is involved in the dormancy of bone metastases, and this transfer appears to occur through gap junctions rather than via EVs (Lim et al., 2011). Whether gap junction-mediated metabolite exchange (Goldberg et al., 1999) occurs between these cells is unknown. TnT-mediated transfer of cytoplasmic material, including mitochondria, to breast cancer cells from bone marrow MSCs and endothelial cells has also been described (Caicedo et al., 2015; Pasquier et al., 2013). The transfer of stroma-derived mitochondria boosts the oxidative metabolism of the cancer cells and promotes their resistance to chemotherapy.
4.2. Prostate cancer metastasis
Prostate cancer is one of the cancers that most frequently metastasizes to bone (Bubendorf et al., 2000). This predilection can anatomically be explained by the close contact between the prostatic venous plexus and the vertebral venous plexus of the spinal column (Batson, 1940). In addition, prostate cancer cells express receptors for cytokines that are abundant in bone marrow, like the CXCL12/CXCR4 or the SCF/cKIT axes, serving as an early homing mechanism (Park et al., 2018).
In contrast to most other tissues, normal prostate epithelial cells are energetically inefficient and perform aerobic glycolysis due to the inhibition of the TCA cycle enzyme m-aconitase, responsible for the synthesis of isocitrate, by high intracellular zinc levels (Elia et al., 2016). This blockade leads to an accumulation of citrate, which is secreted into the prostatic fluid. At the early stages of malignant transformation, prostate cells lose their ability to accumulate zinc due to the downregulation of zinc transporters, relieving the TCA cycle block and allowing the cells to generate energy by citrate oxidation and OXPHOS (Elia et al., 2016). This metabolic reprogramming reduces glucose uptake by prostate cancer cells compared to their non-malignant counterparts, and the mitochondrial metabolism of the cancer cells is fueled instead by lactate released by cancer-associated fibroblasts (Fiaschi et al., 2012). This stromal-derived lactate also supports lipid synthesis by prostate cancer cells (Ippolito et al., 2022), and in accordance overexpression of lipogenic enzymes is an early event in prostate cancer development (Swinnen et al., 2002). Activation of lipid synthesis supports the metabolic requirements of prostate cancer cells and stimulates epigenetic rewiring by providing acetyl moieties for histone acetylation, fostering their growth and metastatic potential (Ippolito et al., 2022). During late stages, prostate cancer cell metabolism is modified again, showing a higher glycolytic phenotype in combination with active OXPHOS (Elia et al., 2016).
Although the metabolism of bone-metastatic prostate cancer cells is still poorly understood, Diedrich and colleagues showed that marrow adipocytes can promote a glycolytic phenotype in the cancer cells, with high expression of glycolytic enzymes and decreased mitochondrial OXPHOS (Fig. 6) (Diedrich et al., 2016). This metabolic phenotype may also be stimulated by the upregulation of HIF signaling that comes with low oxygen levels in the bone. Whitburn et al. recently found that co-cultures of prostate cancer cells with different bone marrow stromal cell lines increased intracellular levels of TCA cycle metabolites in addition to glycolytic metabolites in the cancer cells (Whitburn et al., 2022). In accordance, prostate cancer bone metastasis showed increased expression of genes relating to OXPHOS, the TCA cycle, ROS detoxification, and glutathione metabolism compared to primary prostate cancer. Prostate cancer bone metastasis also showed upregulation of the pentose phosphate pathway (PPP) versus primary tumors. This phenotype was not seen in liver or lung metastases, suggesting that alterations in the PPP are specific to the bone microenvironment and not a generalized metabolic response to metastasis (Whitburn et al., 2022). Mechanistically, bone marrow stromal cells were found to up-regulate the expression of the rate-limiting PPP enzyme G6PD in prostate cancer cells via IL6 secretion. Consequently, blocking G6PD activity inhibited prostate cancer proliferation, migration, mesenchymal phenotype, and increased sensitivity to chemotherapy.
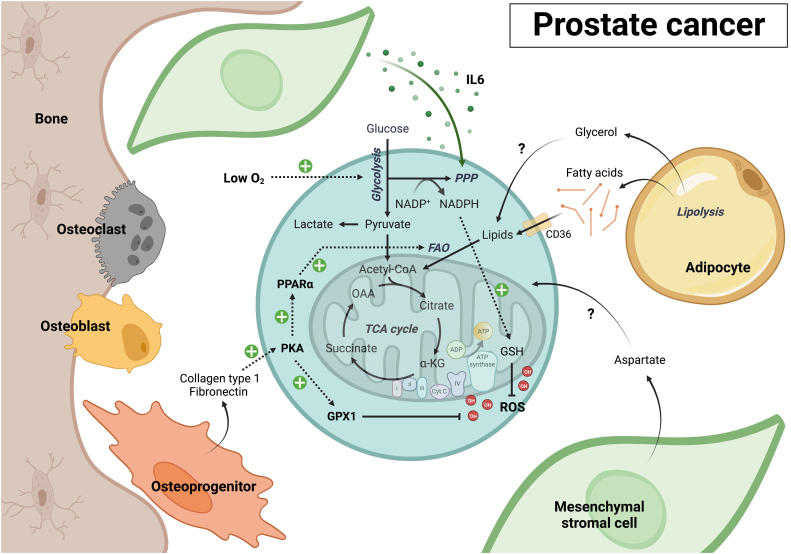
Fig. 6.
Metabolic communication between stromal and prostate cancer cells in bone. Current insights in the metabolic communication occurring in bone-metastatic prostate cancer are very limited in comparison to other cancers growing in the bone marrow. Co-culture with MSCs stimulates glycolysis, TCA cycle activity and redox metabolism in prostate cancer cells. Unique to prostate cancer cells growing in the bone marrow microenvironment is their high rate of PPP activity, a pathway that is stimulated by IL6 released by MSCs. While relatively limited, metastatic prostate cancer cells do exhibit mitochondrial oxidative metabolism, which may be fueled by fatty acids released by bone marrow adipocytes. Adipocytes also release glycerol, although its uptake by and metabolic fate in bone-metastatic prostate cancer cells remains unknown. While no metabolic interactions with mature osteoblasts have been described yet, osteoprogenitors release fibronectin and collagen type 1, which activate protein kinase A (PKA) signaling in prostate cancer cells. PKA increases expression of several metabolic genes in the cancer cells, including some involved in fatty acid metabolism and ROS detoxification. Aspartate, an amino acid highly enriched in the bone marrow, may also fuel mitochondrial metabolism of metastatic prostate cancer cells like it does in primary tumors, although this remains to be investigated.
Since bone-metastatic prostate cancer cells have high rates of glycolysis and the PPP, it is likely that nutrients other than glucose contribute to mitochondrial oxidative metabolism. One possible fuel are fatty acids released by adipocytes, given that prostate cancer bone metastases are rich in free fatty acids and cholesterol compared to normal bone (Thysell et al., 2010), and that exposing adipocytes to prostate cancer cell-conditioned medium upregulates lipolysis (Diedrich et al., 2016). Like breast cancer cells, exposure of prostate cancer cells to adipocyte-conditioned medium induces expression of CD36, FABP4 and several pro-inflammatory cytokines, and prostate bone tumors of mice fed a high-fat diet show similar expressional changes compared to tumors derived from control mice (Herroon et al., 2013). Whether adipocyte-derived fatty acids are used for FAO in prostate cancer cells remains to be confirmed. However, a recent study shows that crosstalk with osteoprogenitors can stimulate fatty acid metabolism in prostate cancer cells (Sanchis et al., 2022). Fibronectin and collagen type 1 released by osteoprogenitors were found to activate protein kinase A signaling in prostate cancer cells, which in turn increased the expression of several metabolic genes, including those encoding PPAR-α (peroxisome proliferator activated receptor alpha), the master transcriptional regulator of FAO, and GPX1 (glutathione peroxidase 1) which plays a crucial role in ROS detoxification. Another possible fuel of metastatic prostate cancer cell mitochondrial metabolism is aspartate, which is highly abundant in bone marrow plasma (van Gastel et al., 2020) and further enriches in prostate cancer bone metastases compared to normal bone (Thysell et al., 2010). Aspartate uptake and metabolism are also known to be increased in prostate cancer cells undergoing EMT (Chen et al., 2020). Further studies are needed to investigate the contribution and relative importance of these and other nutrients for prostate cancer cells growing in the bone marrow microenvironment.
5. Conclusions & future perspectives
In the 45 years since Ray Schofield formulated his hypothesis on the existence of a specialized bone marrow niche for HSCs (Schofield, 1978), our insights into the signals and cellular interactions constituting that niche have grown tremendously, as well as our understanding of the changes that accompany cancer cell invasion (Morrison and Scadden, 2014; Hendriks and Ramasamy, 2020; Ambrosi and Chan, 2021; Comazzetto et al., 2021; Mendez-Ferrer et al., 2020; Pinho and Frenette, 2019; Sanchez-Lanzas et al., 2022). In contrast, the existence of metabolic crosstalk in the normal and malignant bone marrow microenvironment remained under the radar until recently. We now have a much better understanding of the nature and extent of metabolic exchanges and interactions occurring between different cells in the bone marrow. Its importance is particularly evident in cancer, and interference with specific metabolic communication axes on which bone marrow-resident cancer cells depend for their growth and survival may have therapeutic potential, as highlighted throughout this review.
Yet many unanswered questions remain. Most efforts have focused on interactions that affect central carbon metabolism – glycolysis and the TCA cycle – but other areas of cellular metabolism remain poorly studied, such as the metabolism of different lipids, nucleotides, glycans and vitamins. The identity and importance of most metabolite transporters – both importers and exporters – in bone, blood and cancer cells also remain unknown. In addition, even though the metabolic composition of bone marrow plasma is very different than that of peripheral blood plasma, it is unclear whether specific metabolites can serve as homing signals for the bone marrow, and whether this may explain why some tumors, but not others, metastasize to bone. Finally, while a couple of studies have provided the first evidence that metabolic heterogeneity between different bone marrow niches exists, the extent and functional importance of this necessitates further investigation.
Methodologically, most studies of intercellular metabolic interactions are still rudimentary, although technological advances offer hope for improvement. Cellular co-cultures, with or without inserts physically separating the cells, are most used, as is the use of conditioned medium. Combining these systems with mass spectrometry-based metabolomics analysis can reveal interaction-induced changes in the metabolic programs of both cell types, providing clues to potential metabolic cooperation. In this view, the combination of cellular co-cultures with metabolic tracer analysis is of particular interest, as it can be used to label a pathway of interest in one cell type specifically, and then, after tracer removal and initiation of the co-culture, follow the uptake of labeled metabolites by the second cell type (van Gastel et al., 2020). Genetic interference with metabolite transporters offers another elegant way to study metabolic communication. Still, redundancy between transporters can complicate result interpretation, and not all exchanges require a transporter since TnTs or gap junctions can also be involved. Finally, while established cancer cell lines have their merit, the use of primary patient- or animal-derived cells can be much more revealing, as these cells are less adapted to artificial cell culture conditions and therefore often more niche dependent.
The options to study cellular metabolism in vivo, let alone metabolic crosstalk, are even more limited. While metabolomics analysis or in vivo metabolic tracing in cancer cells growing in the bone marrow is definitely feasible, as we have shown in AML mouse models (van Gastel et al., 2020; van Gastel et al., 2021), metabolic analysis of niche cells is much more complicated given their scarcity and lengthy isolation procedures that will impact their metabolic profile. One possible way to overcome these limitations is metabolic imaging. Matrix-assisted laser desorption ionization (MALDI) and desorption electrospray ionization (DESI) mass spectrometry allow the spatial identification of metabolites, and the most advanced systems can now reach single cell or even subcellular resolution (Duenas et al., 2017; Passarelli et al., 2017; Zheng et al., 2022). Using a bioinformatics approach can provide an interesting alternative, as shown by Zheng and colleagues who recently developed a computational tool to infer metabolite-mediated cell-cell communication events based on single-cell RNA-seq data.
Given this technological progress, the extent of cellular metabolism, and the numerous metabolic exchanges that have been described in other tissues such as the brain (Allaman et al., 2011), we are convinced that the coming years will bring a wealth of new insights into the direct and indirect metabolic interactions between bone and blood cells in both the healthy and malignant bone marrow niche.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank all members of the Cellular Metabolism and Microenvironment Laboratory for helpful discussions. We apologize to authors whose work could not be included due to space restrictions. Figures were created with BioRender.com.
Funding
Work in the authors' laboratory is supported by the Belgian Foundation Against Cancer [F/2020/1440, ATE-2022/1863]; the Fund for Research - Biochemical Research on Cancer managed by the King Baudouin Foundation [2021-J1990112-222061]; the Fund for Scientific Research - FNRS [A5/5-CQ/135, M4/1/2/5-MIS/BEJ]; the Walloon Region, within the framework of the strategic axis FRFS-WELBIO [WELBIO-CR-2022 S-08]; the UCLouvain [ADi/IC/15835.2021, ADi/IC/16891.2022, EC/YM/17592.2022]; and the de Duve Institute. H. A. Tirado holds a doctoral research fellowship from the Fund for Research Training in Industry and Agriculture (FRIA) [1.E.027.22], A. Erdem holds a Rubicon postdoctoral research fellowship from the Netherlands Organisation for Health Research and Development (ZonMw) - Netherlands Organisation for Scientific Research (NWO) [2022/14232/ZONMW].
Data availability
No data was used for the research described in the article.
References
- Acar M., et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526(7571):126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal P., et al. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell. 2019;24(5):769–784 e6. doi: 10.1016/j.stem.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I., et al. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34(2):76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Ambrosi T.H., Chan C.K.F. Skeletal stem cells as the developmental origin of cellular niches for hematopoietic stem and progenitor cells. Curr. Top. Microbiol. Immunol. 2021;434:1–31. doi: 10.1007/978-3-030-86016-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber D.A., et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz L., et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- Asada N., et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 2017;19(3):214–223. doi: 10.1038/ncb3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R., et al. Electron transport chain activity is a predictor and target for venetoclax sensitivity in multiple myeloma. Nat. Commun. 2020;11(1):1228. doi: 10.1038/s41467-020-15051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J.E. Sl/Sld hematopoietic progenitors are deficient in situ. Exp. Hematol. 1994;22(2):174–177. [PubMed] [Google Scholar]
- Baryawno N., et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019;177(7):1915–1932 e16. doi: 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson O.V. The function of the vertebral veins and their role in the spread of metastases. Ann. Surg. 1940;112(1):138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister J., et al. Hypoxia-inducible factor 1 (HIF-1) is a new therapeutic target in JAK2V617F-positive myeloproliferative neoplasms. Leukemia. 2020;34(4):1062–1074. doi: 10.1038/s41375-019-0629-z. [DOI] [PubMed] [Google Scholar]
- Benayahu D., et al. Hemopoietic functions of marrow-derived osteogenic cells. Calcif. Tissue Int. 1992;51(3):195–201. doi: 10.1007/BF00334547. [DOI] [PubMed] [Google Scholar]
- Biswas L., et al. Lymphatic vessels in bone support regeneration after injury. Cell. 2023;186(2):382–397 e24. doi: 10.1016/j.cell.2022.12.031. [DOI] [PubMed] [Google Scholar]
- Bloedjes T.A., et al. Metabolic effects of recurrent genetic aberrations in multiple myeloma. Cancers. 2021;13(3) doi: 10.3390/cancers13030396. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D., Yegutkin G.G. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell. 2019;36(6):582–596. doi: 10.1016/j.ccell.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli N., et al. Genomic patterns of progression in smoldering multiple myeloma. Nat. Commun. 2018;9(1):3363. doi: 10.1038/s41467-018-05058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni M., et al. Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: a new attractive target. Blood. 2016;128(5):667–679. doi: 10.1182/blood-2016-01-690743. [DOI] [PubMed] [Google Scholar]
- Boyd A.L., et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat. Cell Biol. 2017;19(11):1336–1347. doi: 10.1038/ncb3625. [DOI] [PubMed] [Google Scholar]
- Bradstock K., et al. Long-term survival and proliferation of precursor-B acute lymphoblastic leukemia cells on human bone marrow stroma. Leukemia. 1996;10(5):813–820. [PubMed] [Google Scholar]
- Bruns I., et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 2014;20(11):1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubendorf L., et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- Burt R., et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134(17):1415–1429. doi: 10.1182/blood.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A., et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci. Rep. 2015;5:9073. doi: 10.1038/srep09073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi L.M., et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Castor A., et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat. Med. 2005;11(6):630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., et al. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 2010;12(6):215. doi: 10.1186/bcr2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., et al. Decreased glucose bioavailability and elevated aspartate metabolism in prostate cancer cells undergoing epithelial-mesenchymal transition. J. Cell. Physiol. 2020;235(7–8):5602–5612. doi: 10.1002/jcp.29490. [DOI] [PubMed] [Google Scholar]
- Chen F., et al. Bone marrow niches in the regulation of bone metastasis. Br. J. Cancer. 2021;124(12):1912–1920. doi: 10.1038/s41416-021-01329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., et al. Oxidative phosphorylation enhances the leukemogenic capacity and resistance to chemotherapy of B cell acute lymphoblastic leukemia. Sci. Adv. 2021;7(11) doi: 10.1126/sciadv.abd6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu M., et al. Myeloma cells deplete bone marrow glutamine and inhibit osteoblast differentiation limiting asparagine availability. Cancers. 2020;12(11) doi: 10.3390/cancers12113267. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu M., et al. ALL blasts drive primary mesenchymal stromal cells to increase asparagine availability during asparaginase treatment. Blood Adv. 2021;5(23):5164–5178. doi: 10.1182/bloodadvances.2020004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clézardin P., et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol. Rev. 2021;101(3):797–855. doi: 10.1152/physrev.00012.2019. [DOI] [PubMed] [Google Scholar]
- Colmone A., et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Comazzetto S., et al. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell. 2021;56(13):1848–1860. doi: 10.1016/j.devcel.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.V., et al. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004;104(9):2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- De Santo C., et al. The arginine metabolome in acute lymphoblastic leukemia can be targeted by the pegylated-recombinant arginase I BCT-100. Int. J. Cancer. 2018;142(7):1490–1502. doi: 10.1002/ijc.31170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M., et al. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat. Cell Biol. 2017;19(6):677–688. doi: 10.1038/ncb3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb G., et al. Hypoxia-inducible factor-1alpha protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk. Res. 2011;35(5):579–584. doi: 10.1016/j.leukres.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Devignes C.-S., et al. Signaling pathways underlying bone metastasis: hypoxia signaling in bone metastasis and beyond. Curr.Mol.Biol.Rep. 2018;4(2):69–79. [Google Scholar]
- Dias A.S., et al. Metabolic crosstalk in the breast cancer microenvironment. Eur. J. Cancer. 2019;121:154–171. doi: 10.1016/j.ejca.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Diedrich J.D., et al. Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1alpha activation. Oncotarget. 2016;7(40):64854–64877. doi: 10.18632/oncotarget.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Morrison S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Donk N.W.C.J., et al. Multiple myeloma. Lancet. 2021;397(10272):410–427. doi: 10.1016/S0140-6736(21)00135-5. [DOI] [PubMed] [Google Scholar]
- Duan C.W., et al. Leukemia propagating cells rebuild an evolving niche in response to therapy. Cancer Cell. 2014;25(6):778–793. doi: 10.1016/j.ccr.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Duarte D., et al. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell. 2018;22(1):64–77 e6. doi: 10.1016/j.stem.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M.E., et al. 3D MALDI mass spectrometry imaging of a single cell: spatial mapping of lipids in the embryonic development of zebrafish. Sci. Rep. 2017;7(1):14946. doi: 10.1038/s41598-017-14949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncavage E.J., et al. Genomic profiling for clinical decision making in myeloid neoplasms and acute leukemia. Blood. 2022;140(21):2228–2247. doi: 10.1182/blood.2022015853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont M., et al. Tunneling nanotubes: intimate communication between myeloid cells. Front. Immunol. 2018;9:43. doi: 10.3389/fimmu.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy F., et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 2015;22(4):577–589. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Egler R.A., et al. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J. Pharmacol. Pharmacother. 2016;7(2):62–71. doi: 10.4103/0976-500X.184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanipour E.A., et al. Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Res. 2013;73(10):2998–3006. doi: 10.1158/0008-5472.CAN-12-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia I., et al. Organ-specific cancer metabolism and its potential for therapy. Handb. Exp. Pharmacol. 2016;233:321–353. doi: 10.1007/164_2015_10. [DOI] [PubMed] [Google Scholar]
- Erdem A., et al. The Glycolytic Gatekeeper PDK1 defines different metabolic states between genetically distinct subtypes of human acute myeloid leukemia. Nat. Commun. 2022;13(1):1105. doi: 10.1038/s41467-022-28737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem A., et al. Inhibition of the succinyl dehydrogenase complex in acute myeloid leukemia leads to a lactate-fuelled respiratory metabolic vulnerability. Nat. Commun. 2022;13(1):2013. doi: 10.1038/s41467-022-29639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler J.T., et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista G.C.M., et al. 4T1 mammary carcinoma colonization of metastatic niches is accelerated by obesity. Front. Oncol. 2019;9:685. doi: 10.3389/fonc.2019.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield H., et al. Myeloma-modified adipocytes exhibit metabolic dysfunction and a senescence-associated secretory phenotype. Cancer Res. 2021;81(3):634–647. doi: 10.1158/0008-5472.CAN-20-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge T., et al. Chemotherapy-resistant human acute myeloid leukemia cells are not enriched for leukemic stem cells but require oxidative metabolism. Cancer Discov. 2017;7(7):716–735. doi: 10.1158/2159-8290.CD-16-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi T., et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72(19):5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- Flores-Figueroa E., et al. In vitro characterization of hematopoietic microenvironment cells from patients with myelodysplastic syndrome. Leuk. Res. 2002;26(7):677–686. doi: 10.1016/s0145-2126(01)00193-x. [DOI] [PubMed] [Google Scholar]
- Forte D., et al. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metab. 2020;32(5):829–843 e9. doi: 10.1016/j.cmet.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., et al. Lactate, a putative survival factor for myeloma cells, is incorporated by myeloma cells through monocarboxylate transporters 1. Exp. Hematol. Oncol. 2015;4:12. doi: 10.1186/s40164-015-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Diez M., et al. Subversion of serotonin receptor signaling in osteoblasts by kynurenine drives acute myeloid leukemia. Cancer Discov. 2022;12(4):1106–1127. doi: 10.1158/2159-8290.CD-21-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen P., et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell. 2019;176(6):1265–1281 e24. doi: 10.1016/j.cell.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gastel N., et al. Induction of a timed metabolic collapse to overcome cancer chemoresistance. Cell Metab. 2020;32(3):391–403. doi: 10.1016/j.cmet.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gastel N., et al. Analysis of leukemia cell metabolism through stable isotope tracing in mice. Bio Protoc. 2021;11(19) doi: 10.21769/BioProtoc.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgievski A., et al. Acute lymphoblastic leukemia-derived extracellular vesicles affect quiescence of hematopoietic stem and progenitor cells. Cell Death Dis. 2022;13(4):337. doi: 10.1038/s41419-022-04761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German N.J., et al. PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2. Mol. Cell. 2016;63(6):1006–1020. doi: 10.1016/j.molcel.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar C.M., et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial I.M., et al. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat. Rev. Clin. Oncol. 2018;15(4):219–233. doi: 10.1038/nrclinonc.2017.197. [DOI] [PubMed] [Google Scholar]
- Giambra V., et al. NOTCH1 promotes T cell leukemia-initiating activity by RUNX-mediated regulation of PKC-theta and reactive oxygen species. Nat. Med. 2012;18(11):1693–1698. doi: 10.1038/nm.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G.S., et al. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat. Cell Biol. 1999;1(7):457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- Hanoun M., et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell. 2014;15(3):365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X., et al. Metabolic imaging reveals a unique preference of symmetric cell division and homing of leukemia-initiating cells in an endosteal niche. Cell Metab. 2019;29(4):950–965 e6. doi: 10.1016/j.cmet.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Hawkins E.D., et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature. 2016;538(7626):518–522. doi: 10.1038/nature19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., et al. Pathobiological pseudohypoxia as a putative mechanism underlying myelodysplastic syndromes. Cancer Discov. 2018;8(11):1438–1457. doi: 10.1158/2159-8290.CD-17-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., et al. Bone marrow niche ATP levels determine leukemia-initiating cell activity via P2X7 in leukemic models. J. Clin. Invest. 2021;131(4) doi: 10.1172/JCI140242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks M., Ramasamy S.K. Blood vessels and vascular niches in bone development and physiological remodeling. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.602278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herroon M.K., et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4(11):2108–2123. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse E., et al. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;5(9) doi: 10.1172/jci.insight.125543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., et al. Understanding the hypoxic niche of multiple myeloma: therapeutic implications and contributions of mouse models. Dis. Model. Mech. 2012;5(6):763–771. doi: 10.1242/dmm.008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippolito L., et al. Lactate rewires lipid metabolism and sustains a metabolic-epigenetic axis in prostate cancer. Cancer Res. 2022;82(7):1267–1282. doi: 10.1158/0008-5472.CAN-21-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkin T., et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532(7599):323–328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto S., et al. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J. Clin. Invest. 2007;117(4):1049–1057. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacamo R., et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-κB mediates chemoresistance. Blood. 2014;123(17):2691–2702. doi: 10.1182/blood-2013-06-511527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian X., et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome. 2020;8(1):74. doi: 10.1186/s40168-020-00854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.M., et al. Metabolic reprogramming of bone marrow stromal cells by leukemic extracellular vesicles in acute lymphoblastic leukemia. Blood. 2016;128(3):453–456. doi: 10.1182/blood-2015-12-688051. [DOI] [PubMed] [Google Scholar]
- Kapp F.G., et al. Protection from UV light is an evolutionarily conserved feature of the haematopoietic niche. Nature. 2018;558(7710):445–448. doi: 10.1038/s41586-018-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J., et al. Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., et al. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr. Relat. Cancer. 2013;20(3):339–348. doi: 10.1530/ERC-12-0398. [DOI] [PubMed] [Google Scholar]
- Kim S., et al. Differential expression of lipid metabolism-related proteins in different breast cancer subtypes. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishton R.J., et al. AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 2016;23(4):649–662. doi: 10.1016/j.cmet.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klco J.M., et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell. 2014;25(3):379–392. doi: 10.1016/j.ccr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkaliaris K.D., Scadden D.T. Cell interactions in the bone marrow microenvironment affecting myeloid malignancies. Blood Adv. 2020;4(15):3795–3803. doi: 10.1182/bloodadvances.2020002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkaliaris K.D., et al. Adult blood stem cell localization reflects the abundance of reported bone marrow niche cell types and their combinations. Blood. 2020;136(20):2296–2307. doi: 10.1182/blood.2020006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., et al. Single-cell resolved imaging reveals intra-tumor heterogeneity in glycolysis, transitions between metabolic states, and their regulatory mechanisms. Cell Rep. 2021;34(7) doi: 10.1016/j.celrep.2021.108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeszinski J.Y., et al. Lipid osteoclastokines regulate breast cancer bone metastasis. Endocrinology. 2017;158(3):477–489. doi: 10.1210/en.2016-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisaki Y., et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong-Lam F., Chi-Fung C.G. Vincristine could partly suppress stromal support to T-ALL blasts during pegylated arginase I treatment. Exp. Hematol. Oncol. 2013;2(1):11. doi: 10.1186/2162-3619-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadinou E.D., et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemma S., et al. MDA-MB-231 breast cancer cells fuel osteoclast metabolism and activity: a new rationale for the pathogenesis of osteolytic bone metastases. Biochim. Biophys. Acta Mol. basis Dis. 2017;1863(12):3254–3264. doi: 10.1016/j.bbadis.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Li S., et al. Somatic mutations drive specific, but reversible,epigenetic heterogeneity states in AML. Cancer Discov. 2020;10(12):1934–1949. doi: 10.1158/2159-8290.CD-19-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P.K., et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- Liu H., et al. Thymidine phosphorylase exerts complex effects on bone resorption and formation in myeloma. Sci. Transl. Med. 2016;8(353):353ra113. doi: 10.1126/scitranslmed.aad8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., et al. Reprogrammed marrow adipocytes contribute to myeloma-induced bone disease. Sci. Transl. Med. 2019;11(494) doi: 10.1126/scitranslmed.aau9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., et al. Osteocyte CIITA aggravates osteolytic bone lesions in myeloma. Nat. Commun. 2022;13(1):3684. doi: 10.1038/s41467-022-31356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., et al. Growth differentiation factor 15 contributes to marrow adipocyte remodeling in response to the growth of leukemic cells. J. Exp. Clin. Cancer Res. 2018;37(1):66. doi: 10.1186/s13046-018-0738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., et al. Stromal-initiated changes in the bone promote metastatic niche development. Cell Rep. 2016;14(1):82–92. doi: 10.1016/j.celrep.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macanas-Pirard P., et al. Bone marrow stromal cells modulate mouse ENT1 activity and protect leukemia cells from cytarabine induced apoptosis. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malard F., Mohty M. Acute lymphoblastic leukaemia. Lancet. 2020;395(10230):1146–1162. doi: 10.1016/S0140-6736(19)33018-1. [DOI] [PubMed] [Google Scholar]
- Marlein C.R., et al. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res. 2019;79(9):2285–2297. doi: 10.1158/0008-5472.CAN-18-0773. [DOI] [PubMed] [Google Scholar]
- Matthijssens F., et al. RUNX2 regulates leukemic cell metabolism and chemotaxis in high-risk T cell acute lymphoblastic leukemia. J. Clin. Invest. 2021;131(6) doi: 10.1172/JCI141566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matula Z., et al. Stromal cells serve drug resistance for multiple myeloma via mitochondrial transfer: a study on primary myeloma and stromal cells. Cancers. 2021;13(14) doi: 10.3390/cancers13143461. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan J.S., Majeti R. The cancer stem cell model: B cell acute lymphoblastic leukaemia breaks the mould. EMBO Mol.Med. 2013;5(1):7–9. doi: 10.1002/emmm.201202207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H., et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824–837. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S., et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S., et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S., et al. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer. 2020;20(5):285–298. doi: 10.1038/s41568-020-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Villanueva D., et al. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFbeta-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011;13(5):R106. doi: 10.1186/bcr3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles L.A., et al. Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature. 2020;587(7834):477–482. doi: 10.1038/s41586-020-2864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.R., et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018;24(7):1036–1046. doi: 10.1038/s41591-018-0052-4. [DOI] [PubMed] [Google Scholar]
- Morita K., et al. Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat. Commun. 2020;11(1):5327. doi: 10.1038/s41467-020-19119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschoi R., et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128(2):253–264. doi: 10.1182/blood-2015-07-655860. [DOI] [PubMed] [Google Scholar]
- Nelson M.A., et al. Intrinsic OXPHOS limitations underlie cellular bioenergetics in leukemia. elife. 2021;10 doi: 10.7554/eLife.63104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre A.R., et al. Bone marrow NG2(+)/Nestin(+) mesenchymal stem cells drive DTC dormancy via TGFbeta2. Nat. Cancer. 2021;2(3):327–339. doi: 10.1038/s43018-021-00179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshiba T., et al. Role of prostaglandin E produced by osteoblasts in osteolysis due to bone metastasis. Biochem. Biophys. Res. Commun. 2003;300(4):957–964. doi: 10.1016/s0006-291x(02)02937-6. [DOI] [PubMed] [Google Scholar]
- Panaroni C., et al. Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins. Blood. 2022;139(6):876–888. doi: 10.1182/blood.2021013832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., et al. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood. 2011;118(8):2191–2199. doi: 10.1182/blood-2011-04-351239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., et al. Bone marrow microenvironment as a regulator and therapeutic target for prostate cancer bone metastasis. Calcif. Tissue Int. 2018;102(2):152–162. doi: 10.1007/s00223-017-0350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier J., et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli M.K., et al. The 3D OrbiSIMS-label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat. Methods. 2017;14(12):1175–1183. doi: 10.1038/nmeth.4504. [DOI] [PubMed] [Google Scholar]
- Passaro D., et al. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell. 2017;32(3):324–341 e6. doi: 10.1016/j.ccell.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pel M., et al. The human and murine hematopoietic stem cell niches: are they comparable? Ann. N. Y. Acad. Sci. 2016;1370(1):55–64. doi: 10.1111/nyas.12994. [DOI] [PubMed] [Google Scholar]
- Peled A., et al. Role of CXCL12 and CXCR4 in the pathogenesis of hematological malignancies. Cytokine. 2018;109:11–16. doi: 10.1016/j.cyto.2018.02.020. [DOI] [PubMed] [Google Scholar]
- Petruk N., et al. CD73 facilitates EMT progression and promotes lung metastases in triple-negative breast cancer. Sci. Rep. 2021;11(1):6035. doi: 10.1038/s41598-021-85379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S., Frenette P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019;20(5):303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S., et al. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev. Cell. 2018;44(5):634–641 e4. doi: 10.1016/j.devcel.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollari S., et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 2011;125(2):421–430. doi: 10.1007/s10549-010-0848-5. [DOI] [PubMed] [Google Scholar]
- Reagan M.R., Ghobrial I.M. Multiple myeloma mesenchymal stem cells: characterization, origin, and tumor-promoting effects. Clin. Cancer Res. 2012;18(2):342–349. doi: 10.1158/1078-0432.CCR-11-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccaro A.M., et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Invest. 2013;123(4):1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- de Rooij B., et al. Tunneling nanotubes facilitate autophagosome transfer in the leukemic niche. Leukemia. 2017;31(7):1651–1654. doi: 10.1038/leu.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshanzamir F., et al. Metastatic triple negative breast cancer adapts its metabolism to destination tissues while retaining key metabolic signatures. Proc. Natl. Acad. Sci. U. S. A. 2022;119(35) doi: 10.1073/pnas.2205456119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier L., et al. Bioactive lipids and cancer metastasis to bone. J. Cancer Metastasis Treat. 2021;7:43. [Google Scholar]
- Saito Y., et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat. Biotechnol. 2010;28(3):275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., et al. Exogenous mitochondrial transfer and endogenous mitochondrial fission facilitate AML resistance to OxPhos inhibition. Blood Adv. 2021;5(20):4233–4255. doi: 10.1182/bloodadvances.2020003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio I., et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Invest. 2010;120(1):142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lanzas R., et al. Diversity in the bone marrow niche: classic and novel strategies to uncover niche composition. Br. J. Haematol. 2022;199(5):647–664. doi: 10.1111/bjh.18355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis P., et al. Bone progenitors pull the strings on the early metabolic rewiring occurring in prostate cancer cells. Cancers. 2022;14(9) doi: 10.3390/cancers14092083. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino A.M., et al. Metabolic adaptation of acute lymphoblastic leukemia to the central nervous system microenvironment is dependent on Stearoyl CoA desaturase. Nat. Cancer. 2020;1(10):998–1009. doi: 10.1038/s43018-020-00115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K., et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13(3):285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- Shafat M.S., et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129(10):1320–1332. doi: 10.1182/blood-2016-08-734798. [DOI] [PubMed] [Google Scholar]
- Shen B., et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591(7850):438–444. doi: 10.1038/s41586-021-03298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., et al. Lipids isolated from bone induce the migration of human breast cancer cells. J. Lipid Res. 2006;47(4):724–733. doi: 10.1194/jlr.M500473-JLR200. [DOI] [PubMed] [Google Scholar]
- Singh B., et al. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26(26):3789–3796. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- Soni S., et al. Molecular insights into the interplay between adiposity, breast cancer and bone metastasis. Clin. Exp. Metastasis. 2021;38(2):119–138. doi: 10.1007/s10585-021-10076-0. [DOI] [PubMed] [Google Scholar]
- Spencer J.A., et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriskanthadevan S., et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood. 2015;125(13):2120–2130. doi: 10.1182/blood-2014-08-594408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen S., et al. Glutamine metabolism in osteoprogenitors is required for bone mass accrual and PTH-induced bone anabolism in male mice. J. Bone Miner. Res. 2021;36(3):604–616. doi: 10.1002/jbmr.4219. [DOI] [PubMed] [Google Scholar]
- Stevens B.M., et al. Fatty acid metabolism underlies venetoclax resistance in acute myeloid leukemia stem cells. Nat. Cancer. 2020;1(12):1176–1187. doi: 10.1038/s43018-020-00126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen J.V., et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int. J. Cancer. 2002;98(1):19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- Tabe Y., et al. Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute monocytic leukemia cells. Cancer Res. 2017;77(6):1453–1464. doi: 10.1158/0008-5472.CAN-16-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman R.S., Emerson S.G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med. 1994;179(5):1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tandon M., et al. Bone metastatic breast cancer cells display downregulation of PKC-zeta with enhanced glutamine metabolism. Gene. 2021;775 doi: 10.1016/j.gene.2021.145419. [DOI] [PubMed] [Google Scholar]
- Tcheng M., et al. Very long chain fatty acid metabolism is required in acute myeloid leukemia. Blood. 2021;137(25):3518–3532. doi: 10.1182/blood.2020008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., et al. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8(1):7. doi: 10.1038/s41408-017-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thysell E., et al. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., et al. Osteocytic connexin hemichannels modulate oxidative bone microenvironment and breast cancer growth. Cancers. 2021;13(24) doi: 10.3390/cancers13246343. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonova A.N., et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569(7755):222–228. doi: 10.1038/s41586-019-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H., et al. Hypoxia-inducible factor-1alpha expression indicates poor prognosis in myelodysplastic syndromes. Leuk. Lymphoma. 2012;53(12):2412–2418. doi: 10.3109/10428194.2012.696637. [DOI] [PubMed] [Google Scholar]
- Tucci J., et al. Adipocytes provide fatty acids to acute lymphoblastic leukemia cells. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.665763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M., et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Hernandez T., et al. HIF-1alpha can act as a tumor suppressor gene in murine acute myeloid leukemia. Blood. 2014;124(24):3597–3607. doi: 10.1182/blood-2014-04-567065. [DOI] [PubMed] [Google Scholar]
- Vilaplana-Lopera N., et al. Crosstalk between AML and stromal cells triggers acetate secretion through the metabolic rewiring of stromal cells. elife. 2022;11 doi: 10.7554/eLife.75908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinado A.C., et al. The bone marrow niche regulates redox and energy balance in MLL::AF9 leukemia stem cells. Leukemia. 2022;36(8):1969–1979. doi: 10.1038/s41375-022-01601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.O., Aspenberg P. Where did bone come from? Acta Orthop. 2011;82(4):393–398. doi: 10.3109/17453674.2011.588861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8(4):399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.H., et al. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158(6):1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J. Hematol. Oncol. 2018;11(1):11. doi: 10.1186/s13045-018-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitburn J., et al. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci. Adv. 2022;8(8) doi: 10.1126/sciadv.abf9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga A.T.J., et al. HIF1/2-exerted control over glycolytic gene expression is not functionally relevant for glycolysis in human leukemic stem/progenitor cells. Cancer Metab. 2019;7:11. doi: 10.1186/s40170-019-0206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski M.T., et al. Mapping and targeting of the leukemic microenvironment. J. Exp. Med. 2020;217(2) doi: 10.1084/jem.20190589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017;8(17):27990–27996. doi: 10.18632/oncotarget.15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yang M.H., et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- Yang R., et al. Conversion of ATP to adenosine by CD39 and CD73 in multiple myeloma can be successfully targeted together with adenosine receptor A2A blockade. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H., et al. Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell. 2016;19(1):23–37. doi: 10.1016/j.stem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V.W., et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J. Exp. Med. 2015;212(5):759–774. doi: 10.1084/jem.20141843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 2019;29(4):966–978 e4. doi: 10.1016/j.cmet.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue R., et al. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18(6):782–796. doi: 10.1016/j.stem.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Zaiss M.M., et al. The gut-bone axis: how bacterial metabolites bridge the distance. J. Clin. Invest. 2019;129(8):3018–3028. doi: 10.1172/JCI128521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhang H., et al. HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119(11):2595–2607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 2012;14(3):276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., et al. 2020. The bone microenvironment invigorates metastatic seeds for further dissemination, bioRxiv. 2020.11.16.383828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., et al. In situ mapping identifies distinct vascular niches for myelopoiesis. Nature. 2021;590(7846):457–462. doi: 10.1038/s41586-021-03201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 2014;20(11):1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- Zheng R., et al. MEBOCOST: Metabolite-mediated Cell Communication Modeling by Single Cell Transcriptome. BioRXiv. 2022 https://www.biorxiv.org/content/10.1101/2022.05.30.494067v1 [Google Scholar]
- Zhou B.O., et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.Z., et al. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene. 2015;34(14):1831–1842. doi: 10.1038/onc.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.Z., et al. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene. 2016;35(43):5597–5607. doi: 10.1038/onc.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.