Abstract
Background
Tumor invasiveness plays a key role in determining surgical strategy and patient prognosis in clinical practice. The study aimed to explore artificial-intelligence-based computed tomography (CT) histogram indicators significantly related to the invasion status of lung adenocarcinoma appearing as part-solid nodules (PSNs), and to construct radiomics models for prediction of tumor invasiveness.
Methods
We identified surgically resected lung adenocarcinomas manifesting as PSNs in Peking University People’s Hospital from January 2014 to October 2019. Tumors were categorized as adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IAC) by comprehensive pathological assessment. The whole cohort was randomly assigned into a training (70%, n=832) and a validation cohort (30%, n=356) to establish and validate the prediction model. An artificial-intelligence-based algorithm (InferRead CT Lung) was applied to extract CT histogram parameters for each pulmonary nodule. For feature selection, multivariate regression models were built to identify factors associated with tumor invasiveness. Logistic regression classifier was used for radiomics model building. The predictive performance of the model was then evaluated by ROC and calibration curves.
Results
In total, 299 AIS/MIAs and 889 IACs were included. In the training cohort, multivariate logistic regression analysis demonstrated that age [odds ratio (OR), 1.020; 95% CI, 1.004–1.037; p=0.017], smoking history (OR, 1.846; 95% CI, 1.058–3.221; p=0.031), solid mean density (OR, 1.014; 95% CI, 1.004–1.024; p=0.008], solid volume (OR, 5.858; 95% CI, 1.259–27.247; p = 0.037), pleural retraction sign (OR, 3.179; 95% CI, 1.057–9.559; p = 0.039), variance (OR, 0.570; 95% CI, 0.399–0.813; p=0.002), and entropy (OR, 4.606; 95% CI, 2.750–7.717; p<0.001) were independent predictors for IAC. The areas under the curve (AUCs) in the training and validation cohorts indicated a better discriminative ability of the histogram model (AUC=0.892) compared with the clinical model (AUC=0.852) and integrated model (AUC=0.886).
Conclusion
We developed an AI-based histogram model, which could reliably predict tumor invasiveness in lung adenocarcinoma manifesting as PSNs. This finding would provide promising value in guiding the precision management of PSNs in the daily practice.
Keywords: lung adenocarcinoma, CT histogram, part-solid nodule, tumor invasiveness, three-dimensional index
Introduction
As the low-dose chest computed tomography (CT) is becoming more popular for lung cancer screening, subsolid nodules (SSNs) are increasingly being detected as an important part of the clinical work of thoracic surgeons (1, 2). SSNs are classified into pure GGN (pGGN) and part-solid nodule (PSN) depending on the presence of solid components (3). Pathologically, adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IAC) can appear on CT images as persistent PSNs (4). The 5-year recurrent-free survival (RFS) after complete resection of AIS and MIA is close to 100%, while the 5-year RFS of stage I IAC is only 74.1% (5). In terms of the extent of surgical resection, sublobar resection, including pulmonary wedge resection and segmentectomy, is mainly recommended for AIS/MIA (6), which can preserve the pulmonary parenchyma and effectively reduce surgery-related complications. Lobectomy and lymph node dissection are required for IAC to pursue a lower tumor recurrence rate with a higher rate of surgical complications. Therefore, the accurate preoperative imaging evaluation of PSN to predict IAC is important to guide clinical treatment decisions, especially for the extent of surgical resection.
In recent years, radiomics had made remarkable progress in identifying the degree of invasiveness in pulmonary lesions suspicious of lung cancer (7, 8). Radiomic parameters only or combined radiomics parameters and clinical features had been utilized to construct prediction models of IAC among SSNs with good diagnostic performance (area under curves, 0.72–0.98) (9–13). Only three studies focused on the assessment of invasiveness among PSN group with a relatively small sample (9, 12, 13). Moreover, most of the above studies used manually time-consuming segmentation, which was difficult to be applied in a real-world setting.
As a convenient and representative method of radiomics, the voxel-based CT histogram could provide some key radiological factors beyond human eyes such as skewness and entropy (7, 14, 15). Recently, voxel-based histogram analysis of chest CT has shown great usefulness in identifying pathological invasiveness, lymph node status prediction, and early-stage lung adenocarcinoma suitable for sublobar resection (16–18). However, several above studies included all radiological types of early-stage lung adenocarcinoma not only SSNs, and several studies about SSNs were limited by a small sample. To our knowledge, no published studies had focused on the part-solid nodule, which needs surgical treatment among SSN subtypes. Furthermore, as a high-efficiency and promising automatic method, artificial intelligence (AI) technology has not been integrated in this diagnostic field to date.
In this study, we proposed to use CT histograms based on novel deep-learning artificial intelligence technology to explore clinical and radiomic indicators that effectively distinguish IAC from AIS/MIA in PSNs with a large sample and to construct a prediction model for the invasiveness of PSNs, which can help guide clinical treatment decisions.
Methods
Study population and selection criteria
This study was a cross-sectional retrospective study. Consecutive patients with chest CT suggestive of PSNs (total diameter, 5–30 mm) and received surgeries at the Department of Thoracic Surgery, Peking University People’s Hospital from January 2014 to October 2019 were included in this study. The determination of PSNs was performed by two experienced radiologists (Qingyi Qi and Yaqi Zeng). Postoperative pathological findings confirmed 1,188 lung nodules as primary lung adenocarcinoma ( Figure 1 ). The corresponding clinical information, pathological information, and imaging information were collected through the electronic medical record system.
Figure 1.
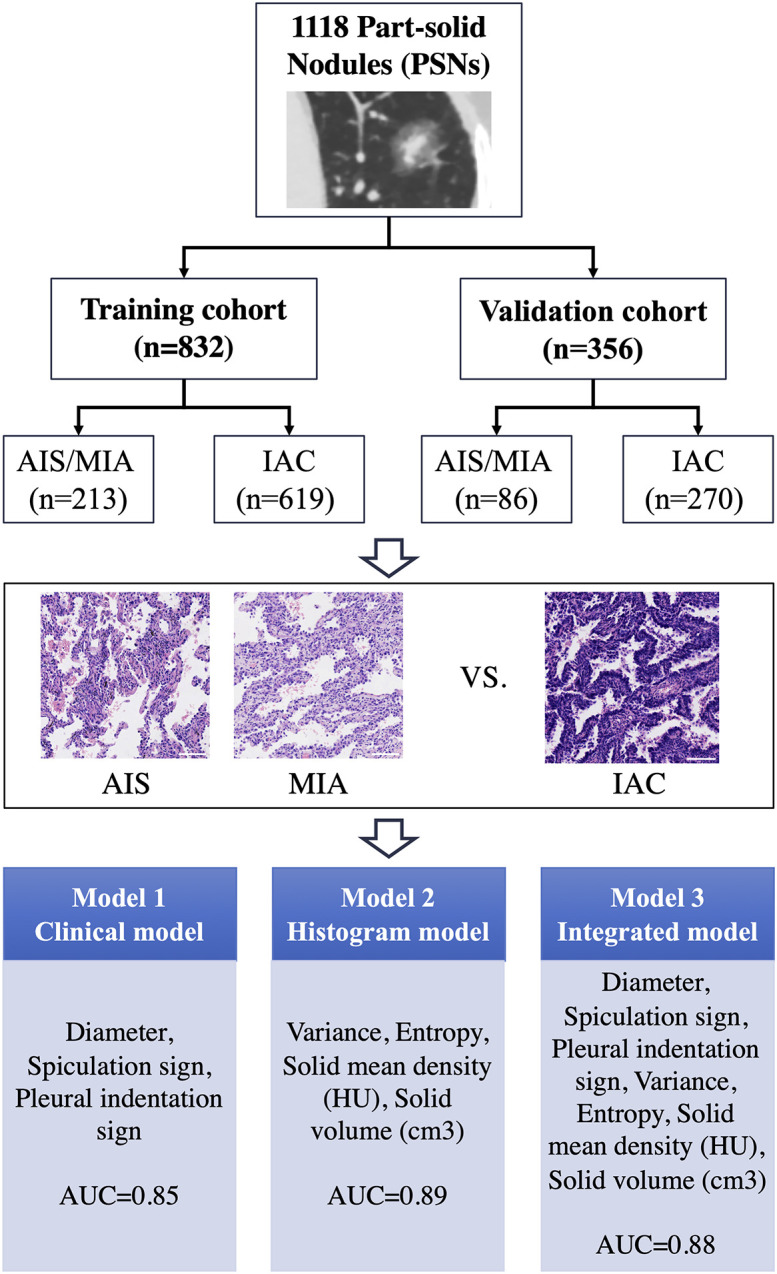
Overall study design process for the training and validation cohorts and prediction performance by each model for predicting invasive adenocarcinoma (IAC). AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; AUC, area under the ROC curve. The length of the ruler in the figure is 10 nm.
CT image acquisition
All patients’ chest CT images were retrospectively collected with the same imaging acquisition parameters: scans with the collimation of 64×0.625 mm, tube voltage of 120 kVp, tube current modulation, gantry rotation speed of 0.5 s/r, and 1.0/1.25 mm reconstructed slice thickness with lung window setting (HU) of (1,600, −600) and mediastinal window (HU) setting of (400, 40) using a 256-row CT scanner (Revolution CT, GE Healthcare, America). All thin-layer images were transmitted to the Lung Nodule Artificial Intelligence Intelligent Assisted Diagnosis System (InferRead CT Lung, Infervision Medical Technology Co., Ltd.) for automatic detection of all lung nodules. The nodules screened by the InferRead system were individually verified by two experienced radiologists, and the PSNs that needed to be surgically resected were manually screened.
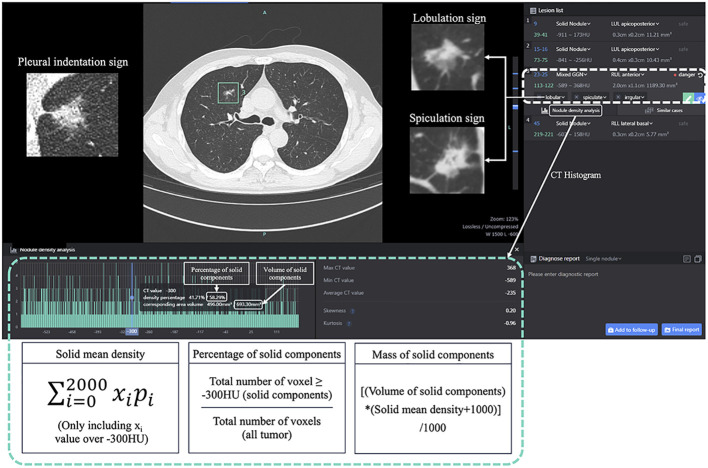
Evaluation of voxel-based histogram features
The lung nodule artificial-intelligence-assisted diagnosis system is based on a deep learning algorithm to achieve automatic segmentation of the range of ground glass nodules and recognition of typical signs (19–21). In the whole calculation process, the system automatically divides the range of the ground glass nodules and calculates the number of voxels corresponding to each CT value in the whole SSN. Each CT value and the corresponding number of voxels are stored as a LIST, and the LIST of the whole nodule is stored as a DICTIONARY. The information obtained is used to calculate the required index by the corresponding formula. First, the CT value threshold of −300 HU was used to distinguish the solid component from the ground glass component. The nodule volume, mean density, solid component volume, percentage of solid component, mass, mass of solid components, and other three-dimensional metrics were calculated based on the voxel method and the corresponding formulas, as follows ( Figure 2 ).
Figure 2.
The CT histogram parameters using InferRead CT Lung, an AI-based pulmonary nodule auxiliary diagnosis system. Mixed GGN, mixed ground-glass nodule.
Solid mean density = (Only including xi ≥ -300HU).
Percentage of solid components= total number of voxel ≥ −300 HU (solid components)/total number of voxels (all tumor)
Mass= [nodule volume×(mean density +1,000)]/1,000.
Mass of solid components= [solid components volume× (solid mean density +1,000)]/1,000.
Then, the CT histograms were constructed based on the number of voxels corresponding to each CT value in the nodule range. Variance, skewness, kurtosis, entropy, and other density histogram-related indicators were automatically calculated by python coding and the corresponding formulae. Meanwhile, the typical signs detected and identified by the system were confirmed by two radiologists as morphological indicators, including lobar signs, spiculation signs, and pleural traction signs.
Construction of the prediction model
The whole cohort was randomly assigned into a training (70%, n=832) and a validation cohort (30%, n=356) to establish and validate the prediction model. This ratio (7:3) ensured the maximal utilization of the data for constructing a predictive model with a considerable number of sample size for validation ( Figure 1 ). Logistical regression analysis based on the training cohort was performed to evaluate the odds ratio (OR) and to assess the parameters’ ability to predicting the risk of IAC. Variables with p-value <0.1 selected in the univariate analysis were included into the multivariate analysis. The variables with significant clinical meanings and the parameters with p-value <0.05 in the multivariate logistical regression analysis were used to establish three predictive models: clinical model (Model 1), histogram model (Model 2), and integrated model (Model 3).
The predictive models were subjected to a 10-fold cross-internal validation within the training cohort and independent validation in the validation cohort. Calibration plots were drawn to evaluate the goodness of fit of models, and the discriminative ability was assessed by receiver operating characteristic (ROC), the area under the curve (AUC), Akaike information criterion (AIC), and Bayesian information criterion (BIC). Higher AUC and lower AIC/BIC values represent higher discriminative ability. Delong’s test was performed to compare the AUCs of different models.
Statistical analysis
Statistical analyses were performed using SPSS 26.0 software and R software version 3.6.0. Categorical variables were analyzed by Pearson chi-square test, continuous variables that conformed to a normal distribution were analyzed by independent samples t-test, and the part that did not conform to a normal distribution was analyzed by rank sum test (Mann–Whitney U test). Variables with p<0.1 in the univariate analysis were included in the multi-factor logistic regression analysis, and the assessment model of IAC in PSN was constructed. The “caret” package in R software was used to depict the calibration plots, and the “pROC” package was utilized to draw the ROC curve, to calculate the AUC, sensitivity, and specificity of the ROC curve, and to perform the Delong’s test. The “stats” package was used to calculate the AIC and BIC values. All statistical tests were two-tailed, and a p-value <0.05 was considered statistically significant.
Results
Clinical characteristics and histopathological nodule features
Among all included PSNs, 30 cases (2.5%) were AIS, 269 cases (22.6%) were MIA, and 889 cases (74.8%) were IAC ( Figure 1 ). Women were the majority (66.5%) of all PSNs. Only a relatively small number of patients had a combined history of smoking (18.5%), previous malignancy (6.8%), and family history of malignancy (12.4%). The mean age of IAC group (60.31) was significantly greater than that of the AIS/MIA group (55.74), and the proportion of smokers was significantly higher than that of the AIS/MIA group. There was no statistical difference between the two groups in terms of previous history of malignancy and family history of malignancy ( Table 1 ).
Table 1.
Comparison of clinical information of AIS/MIA and IAC in 1,188 cases of PSNs.
| Features | AIS/MIA | IAC | p-value |
|---|---|---|---|
| Gender | 0.085 | ||
| Women | 211 (70.6) | 579 (65.1) | |
| Male | 88 (29.4) | 310 (34.9) | |
| Age (years) | 55.74 ± 10.21 | 60.31 ± 9.94 | <0.001 |
| Smoking history | 0.001 | ||
| Non-smokers | 263 (88.0) | 705 (79.3) | |
| Current/previous smokers | 36 (12.0) | 184 (20.7) | |
| History of malignancy | 0.369 | ||
| Yes | 17 (5.7) | 64 (7.2) | |
| None | 282 (94.3) | 825 (92.8) | |
| Family history of malignant tumors | 0.068 | ||
| Yes | 28 (9.4) | 119 (13.4) | |
| None | 271 (90.6) | 770 (86.6) |
Data are expressed as mean ± standard deviation or as a number (percentage).
Imaging features of the different pathological types in PSNs
In assessing the imaging metrics of PSN, we incorporated three-dimensional metrics such as density, volume, mass, and percentage of solid components; morphological metrics such as fractional lobe sign, spiculation sign, and pleural traction sign; and density histogram-related metrics such as variance, kurtosis, skewness, and entropy. The imaging information of the two groups is detailed in Table 2 .
Table 2.
Comparison of imaging features of AIS/MIA and IAC in 1,188 cases of PSNs.
| Features | AIS/MIA | IAC | p-value |
|---|---|---|---|
| Average density (HU) | -483.07 ± 96.11 | -408.40 ± 130.08 | <0.001 |
| Solid mean density (HU) | -212.83 ± 55.16 | -168.19 ± 54.71 | <0.001 |
| Volume (cm3) | 0.57 ± 0.68 | 1.96 ± 1.67 | <0.001 |
| Volume of solid components (cm3) | 0.08 ± 0.17 | 0.59 ± 0.83 | <0.001 |
| Mass (g) | 0.29 ± 0.35 | 1.17 ± 1.08 | <0.001 |
| Mass of solid components (g) | 0.06 ± 0.15 | 0.51 ± 0.75 | <0.001 |
| lobar sign | 105 (35.1) | 685 (77.1) | <0.001 |
| Spiculation sign | 82 (27.4) | 645 (72.6) | <0.001 |
| Pleural traction sign | 4 (1.3) | 215 (24.2) | <0.001 |
| Percentage of solid components (%) | 14.00 ± 14.55 | 28.09 ± 22.55 | <0.001 |
| Density Histogram | |||
| Variance (×10000) | 1.87 ± 1.20 | 2.51 ± 1.23 | < 0.001 |
| Skewness | 0.83 ± 0.49 | 0.69 ± 0.54 | < 0.001 |
| Kurtosis | 3.39 ± 1.50 | 3.09 ± 1.49 | 0.003 |
| Entropy | 7.94 ± 0.55 | 8.63 ± 0.46 | < 0.001 |
Data are expressed as mean ± standard deviation or as a number (percentage).
All imaging features were statistically different between the two groups. Mean density (−408.40 ± 130.08 vs. −483.07 ± 96.11, e<0.001), mean density of the solid component (−168.19 ± 54.71 vs. −212.83 ± 55.16, e<0.001), volume (1.96 ± 1.67 vs. 0.57 ± 0.68, p<0.001), solid component volume (0.59 ± 0.83 vs. 0.08 ± 0.17, p<0.001), mass (1.17 ± 1.08 vs. 0.29 ± 0.35, p<0.001), mass of solid components (0.51 ± 0.75 vs. 0.06 ± 0.15, p<0.001), and percentage of solid components (14.00 ± 14.55 vs. 28.09 ± 22.55, p<0.001) were relatively larger in the IAC group compared to the AIS/MIA group in terms of in the 3D metrics. Among the morphological signs, lobar signs (77.1% vs. 35.1%, p<0.001), spiculation signs (72.6% vs. 27.4%, p<0.001), and pleural traction signs (24.2% vs. 1.3%, p<0.001) were more frequently seen in the IAC group ( Table 2 ). As to the variables of the density histogram, the IAC group had greater values of variance (×10,000) (2.51 ± 1.23 vs. 1.87 ± 1.20, p<0.001) and entropy (8.63 ± 0.46 vs. 7.94 ± 0.55, p<0.001), and relatively smaller skewness (0.69 ± 0.54 vs. 0.83± 0.49, p<0.001) and kurtosis (3.09 ± 1.49 vs. 3.39 ± 1.50, p=0.003) than the AIS/MIA group.
Independent risk factors for IAC in the training cohort
The clinical characteristics and imaging information were balanced in the training and validation cohorts ( Supplementary Table S1 ). The clinical information, 3D imaging metrics, morphological signs, and density histogram-related metrics were included in the multivariate logistic regression analysis. The mass of PSN was calculated based on the mean density and volume. Moreover, there was multi-collinearity between the three-dimensional metrics of PSN as a whole and the solid component, which is generally considered to represent the invasive portion of PSN. In order to avoid the impact of multi-collinearity on the multivariate analysis, the mass-related metrics were excluded from the subsequent analysis, and the three-dimensional metrics of solid component were preferentially selected for the analysis. The results of the multivariate logistic regression analysis showed that solid mean density [odds ratio (OR), 1.015; 95% CI, 1.004–1.027, p=0.009], solid volume (OR, 1.085; 95% CI, 1.028–1.143, p=0.003), diameter (OR, 1.183; 95% CI, 1.085–1.291, p<0.001), variance (OR, 0.605; 95% CI, 0.410–0.893, p=0.011), and entropy (OR, 2.008; 95% CI, 2.750–7.717; p=0.039) were independent risk factors for the pathological invasiveness of PSN as IAC ( Table 3 ).
Table 3.
Univariate and multivariate analyses of the ability of each factor in predicting invasive adenocarcinoma in the training cohort.
| Features | Univariate analysis (input method) | Multivariate analysis (input method) | ||
|---|---|---|---|---|
| OR (95% confidence interval) | p-value | OR (95% confidence interval) | p-value | |
| Gender | 0.157 | |||
| Women | Reference | |||
| Male | 1.276 (0.911-1.788) | |||
| Age (years) | 1.042 (1.025-1.058) | <0.001 | 1.009 (0.990-1.029) | 0.358 |
| Smoking history | 0.006 | 0.087 | ||
| Non-smokers | Reference | Reference | ||
| Current/previous smokers | 1.867 (1.194-2.920) | 1.626 (0.932-2.837) | ||
| Family history of malignant tumors | 0.156 | |||
| Yes | 1.453 (0.867-2.435) | |||
| None | Reference | |||
| Solid mean density (HU) | 1.015 (1.012-1.019) | <0.001 | 1.015 (1.004-1.027) | 0.009 |
| Solid volume (cm3) | 554.326 (122.713-2504.019) | <0.001 | 1.085 (1.028-1.143) | 0.003 |
| lobar sign | <0.001 | 0.933 | ||
| None | Reference | Reference | ||
| Yes | 6.248 (4.463-8.775) | 0.971 (0.487-1.934) | ||
| Spiculation sign | <0.001 | 0.266 | ||
| None | Reference | Reference | ||
| Yes | 6.958 (4.916-9.850) | 1.490 (0.738-3.008) | ||
| Pleural traction sign | <0.001 | 0.263 | ||
| None | Reference | Reference | ||
| Yes | 22.585 (7.121-71.637) | 2.092 (0.574-7.615) | ||
| Diameter (mm) | 1.341 (1.278-1.407) | <0.001 | 1.183 (1.085-1.291) | <0.001 |
| Percentage of solid components (%) | 52.754 (18.592-149.685) | <0.001 | 0.557 (0.033-9.455) | 0.686 |
| CT Histogram | ||||
| Variance (×10,000) | 1.617 (1.370- 1.909) | <0.001 | 0.605 (0.410-0.893) | 0.011 |
| Skewness | 0.624 (0.467-0.833) | 0.001 | 0.447 (0.122-1.6273) | 0.222 |
| Kurtosis | 0.898 (0.817-0.986) | 0.025 | 1.001 (0.717-1.396) | 0.997 |
| Entropy | 13.293 (8.693-20.236) | <0.001 | 2.008 (1.036-3.891) | 0.039 |
Bold values denote statistical significance at the P < 0.05 level.
Optimal model for predicting IAC in PSNs
Three prediction models (clinical, histogram, and integrated model) were built based on the results of the multivariate logistic regression analysis. The clinical model incorporated the diameter of the nodule and CT signs with clinical significance (spiculation sign and pleural traction sign), while the histogram model included AI-derived histogram features, which were independent predictors for the invasion status (variance, entropy, the mean density of the solid component, and volume of the solid component). Finally, we established an integrated model incorporating independent variables in both the clinical and histogram models.
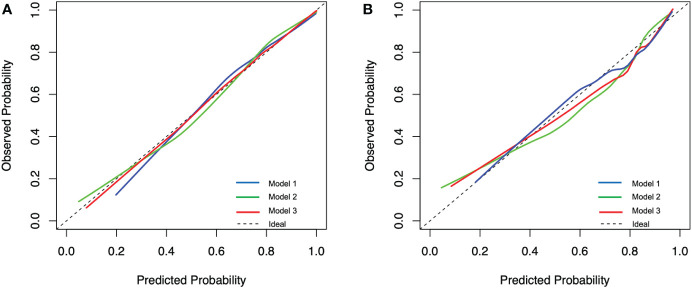
The calibration curves presented good consistency in three models between the predicted and actual observed probability of IAC in the training and validation cohort ( Figure 3 ). The AUCs in the training cohort and validation cohort indicated a better discriminative ability of the histogram model (AUC=0.892) compared with the clinical model (AUC=0.852) and integrated model (AUC=0.886), although the clinical model showed better AIC and BIC values ( Figure 4 ; Table 4 ). Delong’s test showed that the histogram model (model 2) had a significantly higher AUC than the clinical model (p=0.005), while the comparison between histogram model and integrated model fell short of statistical significance (p<0.05, Supplementary Table S2 ).
Figure 3.
Calibration plot of the prediction models for predicting invasive adenocarcinoma lesions from the training cohort (A) and the validation cohort (B). Model 1, clinical model; Model 2, histogram model; Model 3, integrated model.
Figure 4.
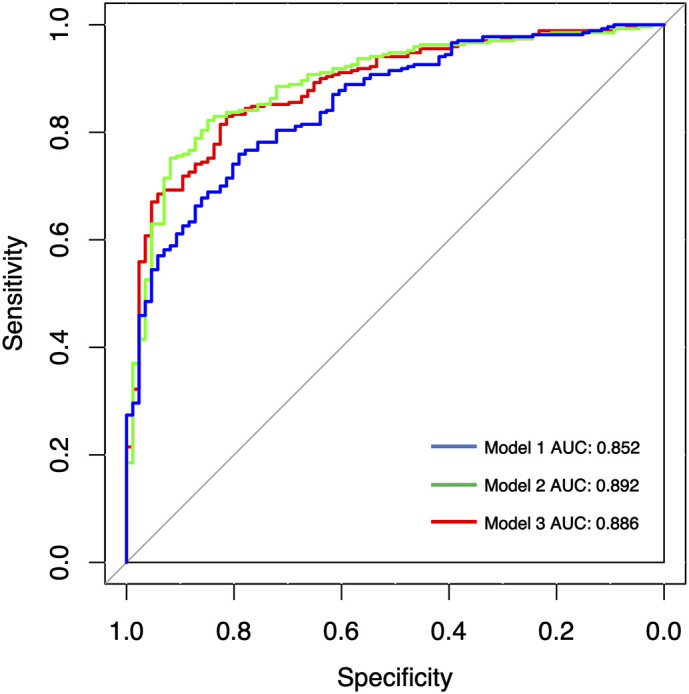
Receiver operating characteristic (ROC) analysis of assessing pathological invasiveness of PSN. AUC, area under the ROC curve; Model 1, clinical model; Model 2, histogram model; Model 3, integrated model.
Table 4.
Diagnostic performance of three predictive models for predicting invasive adenocarcinoma lesions.
| AUC 1 | AUC 2 | AIC | BIC | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Model 1 Clinical model (diameter, spiculation sign, pleural traction sign) |
0.833 | 0.852 | 699.102 | 717.998 | 0.759 | 0.791 |
| Model 2 Histogram model (variance, entropy, the mean density of the solid component, volume of the solid component) |
0.833 | 0.892 | 694.037 | 717.656 | 0.822 | 0.849 |
| Model 3 Integrated model (Clinical model+ Histogram model) |
0.833 | 0.886 | 673.056 | 710.847 | 0.830 | 0.814 |
AUC 1, 10-fold AUC result based on training cohort; AUC 2, results based on validation cohort. AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion.
Discussion
In recent years, the digital processing of medical images becomes a current hot spot. The quantitative analysis of CT information has advanced the understanding of the internal structure of lung nodules. Especially the artificial intelligence technology represented by deep learning has been widely used in the field of medical imaging. The screening, analysis, and diagnosis of lung nodules based on CT images have entered the era of precision and intelligence. The clinical diagnosis process and efficiency of pulmonary nodules have been greatly improved (19, 22). At the same time, AI’s accurate lesion segmentation, lesion volume measurement, and sensitive sign recognition also provide richer features and more reproducible objective indicators for accurate prediction of the pathological invasiveness of lung nodules (23).
In view of the fact that most of the previous reports took SSN as the whole study object, we investigated the clinical and imaging indicators associated with the degree of pathological invasiveness only in PSNs, which are more in need of surgical intervention. The multivariate analysis revealed that diameter, solid mean density, solid volume, variance, and entropy were independent risk factors of IAC in the training cohort. Furthermore, we constructed three prediction models of IAC using clinical information, AI-based density histogram features, and both from a large sample cohort. The histogram model constructed by AI-based density histogram features showed the best performance in assessing the pathological invasiveness of PSNs in the validation cohort (AUC=0.892). The possible reason that the integrated model has lower AUC value than the histogram model is that the addition of morphological signs may reduce the specificity.
In the selection of the differentiation threshold between solid and ground glass components, previous studies found that the sensitivity of solid component recognition gradually decreases and the specificity gradually increases with the gradual increase in the set threshold in a certain range, while the recognition effect of the ground glass component is the opposite. One of the thresholds with a better integrative performance of solid component recognition is −300 HU (18, 24). Therefore, −300 HU was used as the threshold for the classification of ground glass components and solid components in this study.
Considering that the solid component of PSN tends to correspond to the fraction of histological invasive growth, we preferentially selected the solid component indicators in the multivariate logistic regression analysis. Yu et al. from Korea suggested that the assessment effect of high-dimensional indicators was better than that low-dimensional indicators (3D > 2D > 1D) in the assessment of pathological invasiveness in the PSN (25). Many previous studies have demonstrated the correlation between the diameter of the solid component or CTR and the pathological invasiveness of PSNs (26, 27). However, there are still relatively few studies on the correlation between three-dimensional metrics of PSN and the degree of pathological invasiveness. One study reported that mass and volume were independent factors for IAC in PSNs, but this study did not evaluate metrics such as mean density of solid components and mass of solid components (28). In the present study, the volume, mean density, and mass of both the total nodule and solid component were investigated. The volume and mean density of the solid component were independent risk factors of IAC after ruling out of the mass index to avoid the impact of multi-collinearity on the multivariate analysis. Because of the comprehensive nature of 3D metrics, especially 3D metrics of solid component, they can reflect the overall nature of nodules more comprehensively and are good indicators for assessing pathological invasiveness of PSNs.
The CT histogram metrics have undergone a gradual transition from the number and size of peaks to the integrated calculation metrics. We calculated the four most frequently used composite metrics, variance, skewness, kurtosis, and entropy to describe the distribution of the overall CT values of PSNs. The results of our study showed that although all four metrics were associated with the degree of pathological invasiveness in PSN, only variance (OR, 0.605; 95% CI, 0.410–0.893, p=0.011) and entropy (OR, 2.008; 95% CI, 2.750–7.717; p=0.039) were independent risk factors for IAC. Most of the current studies on CT histogram indicators and the degree of pathological invasiveness of SSN did not distinguish pGGN and PSN, with SSN as a whole study. Yagi et al. also showed that AIS/MIA had significantly higher skewness and kurtosis and relatively lower values of variance and entropy compared to IAC, and the results of multivariate logistic regression analysis showed that entropy was an independent risk factor between the two groups (29). However, when Chae et al. explored imaging metrics for assessing invasiveness in PSNs, they found that a higher kurtosis was an independent factor in distinguishing pre-invasive lesions (AAH/AIS) from invasive lesions (MIA/IAC) (9). It follows that less invasive lesions tend to show lower variance and entropy and greater kurtosis and skewness in the univariate analysis, representing lower heterogeneity and a more concentrated state at lower CT values. Entropy and variance are important indicators for differentiating pathological invasiveness in PSNs, but the grouping of pathological types expected to be differentiated should also be considered.
Beyond the first-order histogram features, the second- and higher-order texture features extracted by radiomics techniques were also integrated in the assessment of invasiveness among SSNs (30–32). Li et al. obtained the CT texture features of 109 SSNs and found that the surface area feature and the extruded surface area feature could be predictors of IACs compared with MIAs (33). Wu et al. analyzed the association between CT−based conventional features/selected radiomic features and histological invasiveness of 203 SSNs in the training cohort and 57 SSNs in the validation cohort. The diagnostic performance of the radiomic feature was as great as that of quantitative CT feature (nodular size and solid component) (34). Recently, they further studied the radiomics features of 260 SSNs and constructed a LASSO-derived model integrating semantic–radiomic features, which showed excellent diagnosis performance (AUC, 0.957) to predict invasive SSNs (35).
In the majority of above studies, the SSNs were investigated as a whole, while only three studies focused on the assessment of invasiveness in PSNs by radiomics technology. Chae et al. constructed a three-layered artificial neural networks model with mean attenuation, standard deviation of attenuation, mass, kurtosis, and entropy in 86 PSNs (9). Even though the model showed excellent performance in differentiation of preinvasive lesions from IACs (AUC, 0.981), the small sample and lack of validation limited the model’s application. Weng et al. identified that four radiomics features, including MaxIntensity, RMS, ZonePercentage, and Long-RunEmphasis_angle0_offset7, were the best discriminators to predict invasiveness of PSNs (12). A nomogram that integrated lesion shape and radiomic signature could achieve a satisfactory AUC of 0.888 (12). In a retrospective multicenter study that included 297 PSNs, Wu et al. extracted radiomic features from the different regions [gross tumor volume (GTV), solid, ground-glass, and perinodular] (13). The radiomics model based on ground-glass and solid features yielded an AUC of 0.98 on the test data set, which was significantly higher compared with the Brock, clinical–semantic features, and volumetric models. However, the model required ground-glass and solid CT radiomic features, which was too complicated to apply in clinical practice.
Nevertheless, the above studies mostly used the manual delineation method for ROI delineation and segmentation, which was time consuming and prone to inter-observer inconsistency in a real-world setting (8). Deep-learning AI techniques, as a high-efficiency and promising automatic method, has been increasingly applied in automatic lesion delineation to accelerate radiomics pipeline (32, 36, 37). In the comparison of the clinical model, histogram model, and integrated model, the histogram model constructed by AI-based histogram features demonstrated the best performance in the validation cohort (AUC=0.892), while the integrated model did not achieve better diagnostic efficiency compared with the histogram model (p=0.34, Delong’s test). As the histogram features could be calculated automatically by the deep-learning AI software (InferRead CT Lung), surgeons could apply the histogram model for surgical planning of PSNs in the real-world clinical practice after embedding this software in the medical imaging system.
This study has some limitations. First, this study is a retrospective single-center study with selective bias and no external validation. Second, the solid component diameter and CTR were not included in the analysis considering the measurement of solid component diameter is inherently subjective. Third, only the first order radiomic features, no more in-depth second or third radiomic features, were studied this time. There is still much room for improvement in the diagnostic performance of our model. However, the more complex radiomic features make it more difficult in practical clinical application.
Conclusion
This large sample study demonstrated that AI-based CT histogram features could assess the pathological invasiveness of PSNs accurately. With further external validation in the future, this convenient histogram model is very promising to be applied to guide clinical treatment decisions of PSNs in the real-world setting.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Peking University People’s Hospital ethics committee (2022PHB011-002). Written informed consent for participation was not required for this study by national legislation and institutional requirements.
Author contributions
JG, QQ, HL conceived and designed the study. ZW, ZS, SC, JY, YZ and NH performed the experiments. ZW, ZS, DW and HW analyzed the data. FY, XL, YL provided critical inputs on design, analysis, and interpretation of the study. All the authors had access to the data. All authors read and approved the final manuscript as submitted.
Funding Statement
The work is funded by the Major Research Plan of National Natural Science of China (grant 92259303, 92059203), National Natural Science Foundation of China (82002410) and Peking University People's Hospital Scientific Research Development Funds (RS2022-05, RDJP2022-27).
Conflict of interest
Authors DW and HW were employed by Infervision Medical Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1096453/full#supplementary-material
References
- 1. Pedersen JH, Saghir Z, Wille M, Thomsen LH, Ashraf H. Ground-glass opacity lung nodules in the era of lung cancer CT screening: Radiology, pathology, and clinical management. Oncology (2016) 30(3):266. [PubMed] [Google Scholar]
- 2. Wu FZ, Huang YL, Wu CC, Tang EK, Chen CS, Mar GY, et al. Assessment of selection criteria for low-dose lung screening CT among Asian ethnic groups in Taiwan: From mass screening to specific risk-based screening for non-smoker lung cancer. Clin Lung Cancer (2016) 17(5):e45–56. doi: 10.1016/j.cllc.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 3. Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: A statement from the Fleischner Society. Radiology (2013)266(1):304–17. doi: 10.1148/radiol.12120628 [DOI] [PubMed] [Google Scholar]
- 4. Gao JW, Rizzo S, Ma LH, Qiu XY, Warth A, Seki N, et al. Pulmonary ground-glass opacity: Computed tomography features, histopathology and molecular pathology. Transl Lung Cancer Res (2017) 6(1):68–75. doi: 10.21037/tlcr.2017.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu S, Rui W, Yang Z, Yuan L, Sun Y. Precise diagnosis of intraoperative frozen section is an effective method to guide resection strategy for peripheral small-sized lung adenocarcinoma. J Clin Oncol (2016) 34(4):307. doi: 10.1200/JCO.2015.63.4907 [DOI] [PubMed] [Google Scholar]
- 6. Dembitzer FR, Flores RM, Parides MK, Beasley MB. Impact of histologic subtyping on outcome in lobar vs sublobar resections for lung cancer: a pilot study. Chest (2014) 146(1):175–81. doi: 10.1378/chest.13-2506 [DOI] [PubMed] [Google Scholar]
- 7. Eriguchi D, Shimada Y, Imai K, Furumoto H, Okano T, Masuno R, et al. Predictive accuracy of lepidic growth subtypes in early-stage adenocarcinoma of the lung by quantitative CT histogram and FDG-PET. Lung Cancer (2018) 125:14–21. doi: 10.1016/j.lungcan.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 8. Yang CC, Chen CY, Kuo YT, Ko CC, Wu WJ, Liang CH, et al. Radiomics for the prediction of response to antifibrotic treatment in patients with idiopathic pulmonary fibrosis: A pilot study. Diagn (Basel) (2022) 12(4):1002. doi: 10.3390/diagnostics12041002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chae HD, Park CM, Park SJ, Lee SM, Kim KG, Goo JM. Computerized texture analysis of persistent part-solid ground-glass nodules: differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology (2014) 273(1):285. doi: 10.1148/radiol.14132187 [DOI] [PubMed] [Google Scholar]
- 10. Zhao W, Xu Y, Yang Z, Sun Y, Li C, Jin L, et al. Development and validation of a radiomics nomogram for identifying invasiveness of pulmonary adenocarcinomas appearing as subcentimeter ground-glass opacity nodules. Eur J Radiol (2019) 112:161–8. doi: 10.1016/j.ejrad.2019.01.021 [DOI] [PubMed] [Google Scholar]
- 11. Xue X, Yang Y, Huang Q, Cui F, Lian Y, Zhang S, et al. Use of a radiomics model to predict tumor invasiveness of pulmonary adenocarcinomas appearing as pulmonary ground-glass nodules. BioMed Res Int (2018) 2018:6803971. doi: 10.1155/2018/6803971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weng Q, Zhou L, Wang H, Hui J, Chen M, Pang P, et al. A radiomics model for determining the invasiveness of solitary pulmonary nodules that manifest as part-solid nodules. Clin Radiol (2019) 74(12):933–43. doi: 10.1016/j.crad.2019.07.026 [DOI] [PubMed] [Google Scholar]
- 13. Wu G, Woodruff HC, Shen J, Refaee T, Sanduleanu S, Ibrahim A, et al. Diagnosis of invasive lung adenocarcinoma based on chest CT radiomic features of part-solid pulmonary nodules: A multicenter study. Radiology (2020) 297(2):E282. doi: 10.1148/radiol.2020209019 [DOI] [PubMed] [Google Scholar]
- 14. Bak SH, Lee HY, Kim JH, Um SW, Kwon OJ, Han J, et al. Quantitative CT scanning analysis of pure ground-glass opacity nodules predicts further CT scanning change. Chest (2016) 149(1):180–91. doi: 10.1378/chest.15-0034 [DOI] [PubMed] [Google Scholar]
- 15. Ikeda K, Awai K, Mori T, Kawanaka K, Yamashita Y, Nomori H. Differential diagnosis of ground-glass opacity nodules: CT number analysis by three-dimensional computerized quantification. Chest (2007) 132(3):984–90. doi: 10.1378/chest.07-0793 [DOI] [PubMed] [Google Scholar]
- 16. Qiu ZB, Zhang C, Chu XP, Cai FY, Yang XN, Wu YL, et al. Quantifying invasiveness of clinical stage IA lung adenocarcinoma with computed tomography texture features. J Thorac Cardiovasc Surg (2022) 163(3):805–815.e3. doi: 10.1016/j.jtcvs.2020.12.092 [DOI] [PubMed] [Google Scholar]
- 17. Shimada Y, Kudo Y, Furumoto H, Imai K, Maehara S, Tanaka T, et al. Computed tomography histogram approach to predict lymph node metastasis in patients with clinical stage IA lung cancer. Ann Thorac Surg (2019) 108(4):1021–8. doi: 10.1016/j.athoracsur.2019.04.082 [DOI] [PubMed] [Google Scholar]
- 18. Yoshiyasu N, Kojima F, Hayashi K, Bando T. Radiomics technology for identifying early-stage lung adenocarcinomas suitable for sublobar resection. J Thorac Cardiovasc Surg (2021) 162(2):477–485.e1. doi: 10.1016/j.jtcvs.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Yan F, Lu X, Zheng G, Zhang B. IILS: Intelligent imaging layout system for automatic imaging report standardization and intra-interdisciplinary clinical workflow optimization. EBioMedicine (2019) 44:162–81. doi: 10.1016/j.ebiom.2019.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang K, Liu J, Tang W, Zhang H, Wu J. Identification of benign and malignant pulmonary nodules on chest CT using improved 3D U-net deep learning framework. Eur J Radiol (2020) 129:109013. doi: 10.1016/j.ejrad.2020.109013 [DOI] [PubMed] [Google Scholar]
- 21. Zhu Y, Liu YL, Feng Y, Yang XY, Zhang J, Chang D, et al. A CT-derived deep neural network predicts for programmed death-1 ligand-1 (PD-L1) expression status in advanced lung adenocarcinomas (LUAD). Social Science Electronic Publishing. Ann Transl Med (2020) 8(15):930. doi: 10.21037/atm-19-4690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu K, Li Q, Ma J, Zhou Z, Sun M, Deng Y, et al. Evaluating a fully automated pulmonary nodule detection approach and its impact on radiologist performance. Radiol Artif Intell (2019) 1(3):e180084. doi: 10.1148/ryai.2019180084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu Y, Li Y, Yin H, Tang W, Fan G. Consecutive serial non-contrast CT scan-based deep learning model facilitates the prediction of tumor invasiveness of ground-glass nodules. Front Oncol (2021) 11:725599. doi: 10.3389/fonc.2021.725599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scholten ET, Jacobs C, Van Ginneken B, Van Riel S, Vliegenthart R, Oudkerk M, et al. Detection and quantification of the solid component in pulmonary subsolid nodules by semiautomatic segmentation. Eur Radiol (2015) 25(2):488–96. doi: 10.1007/s00330-014-3427-z [DOI] [PubMed] [Google Scholar]
- 25. Yu WS, Hong SR, Lee JG, Lee JS, Jung HS, Kim DJ, et al. Three-dimensional ground glass opacity ratio in CT images can predict tumor invasiveness of stage IA lung cancer. Yonsei Med J (2016) 57(5):1131–8. doi: 10.3349/ymj.2016.57.5.1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yue X, Liu S, Liu S, Yang G, Li Z, Wang B, et al. HRCT morphological characteristics distinguishing minimally invasive pulmonary adenocarcinoma from invasive pulmonary adenocarcinoma appearing as subsolid nodules with a diameter of ≤3 cm. Clin Radiol (2018) 73(4):411.e7–411.e15. doi: 10.1016/j.crad.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 27. Sun F, Xi J, Zhan C, Yang X, Wang L, Shi Y. Ground glass opacities: Imaging, pathology, and gene mutations. J Thorac Cardiovasc Surg (2018) 156(2):808–13. doi: 10.1016/j.jtcvs.2018.02.110 [DOI] [PubMed] [Google Scholar]
- 28. Wang XW, Chen WF, He WJ, Yang ZM, Li M, Xiao L, et al. CT features differentiating pre- and minimally invasive from invasive adenocarcinoma appearing as mixed ground-glass nodules: Mass is a potential imaging biomarker. Clin Radiol (2018) 73(6):549–54. doi: 10.1016/j.crad.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 29. Yagi T, Yamazaki M, Ohashi R, Ogawa R, Ishikawa H, Yoshimura N, et al. HRCT texture analysis for pure or part-solid ground-glass nodules: distinguishability of adenocarcinoma in situ or minimally invasive adenocarcinoma from invasive adenocarcinoma. Japanese J Radiol (2018) 2(36):113–21. doi: 10.1007/s11604-017-0711-2 [DOI] [PubMed] [Google Scholar]
- 30. Wu YJ, Wu FZ, Yang SC, Tang EK, Liang CH. Radiomics in early lung cancer diagnosis: From diagnosis to clinical decision support and education. Diagn (Basel) (2022) 12(5):1064. doi: 10.3390/diagnostics12051064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang L, Zhang H, Lei H, Zhou H, Wu Y, Shen J. Diagnosis of benign and malignant pulmonary ground-glass nodules using computed tomography radiomics parameters. Technol Cancer Res Treat (2022) 21:15330338221119748. doi: 10.1177/15330338221119748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kao TN, Hsieh MS, Chen LW, Yang CJ, Chuang CC, Chiang XH, et al. CT-based radiomic analysis for preoperative prediction of tumor invasiveness in lung adenocarcinoma presenting as pure ground-glass nodule. Cancers (Basel) (2022) 14(23):5888. doi: 10.3390/cancers14235888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li W, Wang X, Zhang Y, Li X, Li Q, Ye Z. Radiomic analysis of pulmonary ground-glass opacity nodules for distinction of preinvasive lesions, invasive pulmonary adenocarcinoma and minimally invasive adenocarcinoma based on quantitative texture analysis of CT. Chin J Cancer Res (2018) 30(4):415–24. doi: 10.21147/j.issn.1000-9604.2018.04.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu YJ, Liu YC, Liao CY, Tang EK, Wu FZ. A comparative study to evaluate CT-based semantic and radiomic features in preoperative diagnosis of invasive pulmonary adenocarcinomas manifesting as subsolid nodules. Sci Rep (2021) 11(1):66. doi: 10.1038/s41598-020-79690-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu F-Z, Wu Y-J, Tang E-K. An integrated nomogram combined semantic-radiomic features to predict invasive pulmonary adenocarcinomas in subjects with persistent subsolid nodules. Quantit Imaging Med Surg (2022) 13(2):654–68. doi: 10.21037/qims-22-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang CH, Liu YC, Wan YL, Yun CH, Wu WJ, Lopez-Gonzalez R, et al. Quantification of cancer-developing idiopathic pulmonary fibrosis using whole-lung texture analysis of HRCT images. Cancers (Basel) (2021) 13(22):5600. doi: 10.3390/cancers13225600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen X, Qi Q, Sun Z, Wang D, Sun J, Tan W, et al. Total nodule number as an independent prognostic factor in resected stage III non-small cell lung cancer: a deep learning-powered study. Ann Transl Med (2022) 10(2):33. doi: 10.21037/atm-21-3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.