Abstract
We present a case where Hyphopichia burtonii, a yeast, speciated from peritoneal fluid in a cirrhotic patient with secondary peritonitis. The patient, a man in his 60s with decompensated cirrhosis, was admitted for an upper gastrointestinal (GI) bleed. On admission, he was treated empirically for spontaneous bacterial peritonitis (SBP) but failed to improve with antibiotics. Serial paracenteses revealed polymicrobial peritonitis and rising peritoneal polymorphonuclear leukocytes (PMNs). These findings raised concerns for secondary peritonitis, prompting an abdominal computed tomography (CT) scan which revealed ischemic bowel. Among the peritoneal microbiota isolated, Hyphopichia burtonii predominated. Hyphopichia burtonii has only recently been reported as a human pathogen, previously it had only reported as a pathogen in bats[1,2].
Keywords: Mycology, Peritonitis, Intensive care unit, Cirrhosis
Highlights
-
•
Patients with cirrhosis and ascites should have a diagnostic paracentesis performed when hospitalized
-
•
Sgns of secondary peritonitis include polymicrobial peritonitis, a poor response to antibiotics, and increasing PMNs on serial paracenteses.
-
•
Secondary peritonitis differs from spontaneous peritonitis based on presence of a surgically treatable abdominal source; mortality is nearly 100% without surgical intervention.
-
•
Hyphopichia burtonii has now been documented twice as a human pathogen; the implications of this finding require further investigation.
Background
This case highlights the diagnostic importance of delineating spontaneous from secondary peritonitis in cirrhotic patients and provides an overview of both bacterial and fungal peritonitis. While patients with cirrhosis are at increased risk of infection, up to one-third with bacterial infections may be otherwise asymptomatic [3]. Any patient with cirrhosis and ascites who is hospitalized emergently should have a diagnostic paracentesis performed without delay [3]. This case of secondary peritonitis due to intestinal infarction represents the second case report of Hyphopichia burtonii, a fungus, as a human pathogen. Ubiquitous in nature, Hyphopichia burtonii is best known as a yeast responsible for food spoilage, often inhabiting fermented baked products with high sugar and low water content [4]. Hyphopichia burtonii is also isolated in dairy products, cured meat, beetles, and plants such as cacti [4].
Case presentation
A man in his 60s with decompensated alcoholic cirrhosis, prior episodes of upper GI hemorrhage, ascites, and hepatic encephalopathy presented to a community hospital with altered mental status and upper GI bleeding (Fig. 1). He underwent esophagogastroduodenoscopy (EGD) with 7 variceal bands applied. He was treated empirically for SBP with the antibiotic piperacillin-tazobactam. He was also found to have acute kidney injury, presumable secondary to hepatorenal syndrome (HRS), and treated with midodrine, octreotide and albumin.
Fig. 1.
Hospital Course.
Notably, three years prior to this admission, the patient presented to the same community hospital with a strangulated umbilical hernia which was repaired successfully via laparotomy incision. One year later, he developed an incisional hernia; revision was attempted laparoscopically by the same surgeon but was aborted due to the presence of large volume ascites. While recovering from the variceal bleed during this present admission, on hospital day (HD) 3 the patient was noted to have a persistently distended abdomen. Computed tomography (CT) of the abdomen demonstrated ascites and multiple loops of small bowel within the ventral hernia with decompressed bowel exiting the hernia sac. General surgery was consulted for consideration of surgical intervention. The patient’s outpatient surgeon evaluated the patient and declined surgical intervention, given the risk of recurrence of the hernia, low suspicion for bowel obstruction given the presence of bowel movements, and concern that the risk of operative intervention would outweigh the benefits.
Consistent with 2021 American Association for the Study of Liver Diseases (AASLD) SBP and HRS Guidelines [3], a paracentesis was performed on HD 3, soon after identification of ascites on CT imaging, with ascitic fluid demonstrating 4046 white blood cells (WBC) per cubic millimeter (mm3), 96% polymorphonuclear leukocytes (PMNs), and culture positive for Klebsiella pneumoniae and Enterobacter cloacae resistant to piperacillin-tazobactam. The patient was transitioned from piperacillin-tazobactam to meropenem.
On HD 7, the patient underwent another paracentesis, with 8.3 L of ascitic fluid drained, and on HD 9 was transferred to our hospital for a higher level of care. On arrival, he had normal vital signs, with West Haven grade III encephalopathy, a large ventral hernia, and a soft, nontender abdomen with large ascites. At time of hospital transfer, notable hematology and biochemistry results included serum WBC 25,600/mm3, creatinine (Cr) 2.07 mg/dL, and a Model for End Stage Liver Disease-Sodium (MELD-Na) score of 24. On HD 10, a repeat paracentesis was performed, with 4.0 L ascitic fluid drained, with notable hematology and microbiological results including WBC 11,385/mm3 with 95% PMNs and culture grain stain with budding yeast. Due to concern for fungal peritonitis, micafungin was initiated while meropenem was continued. The fungal culture from HD 10 later speciated to pan-sensitive Candida guilliermondii. Despite sensitivity to other antifungals, micafungin was continued due worsening mental status and septic shock. On HD 13 he was transferred to the intensive care unit (ICU) where he was intubated and initiated on vasopressors. At the time of ICU transfer, pertinent hematology and biochemistry results included WBC 26,800/mm3, blood urea nitrogen (BUN) 98 mg/dL and Cr 4.09 mg/dL. Paracentesis performed on HD 13 yielded 2.2 L of frank pus, with ascitic fluid WBC 18,975/mm3 (differential not performed due to degraded WBCs). Microscopy of the peritoneal fluid demonstrated mixed microbiota with budding yeast and gram-positive cocci (GPC) in pairs and chains (tracheal, urine, and blood cultures were negative). Due to the presence of GPC, daptomycin was initiated. A CT scan of the abdomen performed on HD 13 demonstrated ischemic and perforated bowel within the hernia (Fig. 2).
Fig. 2.
Abdominopelvic CT scan on hospital day 13 revealing a large complex ventral abdominal hernia (A) containing multiple loops of distal small bowel. The indistinctness of the bowel wall with pneumatosis and adjacent free air are concerning for ischemic bowel with perforation.
Differential diagnosis
While the ascitic fluid on HD 3 suggested spontaneous bacterial peritonitis, the patient’s lack of improvement, combined with the development of polymicrobial peritonitis and ischemic bowel within the hernia favored the diagnosis of secondary peritonitis.
Outcome
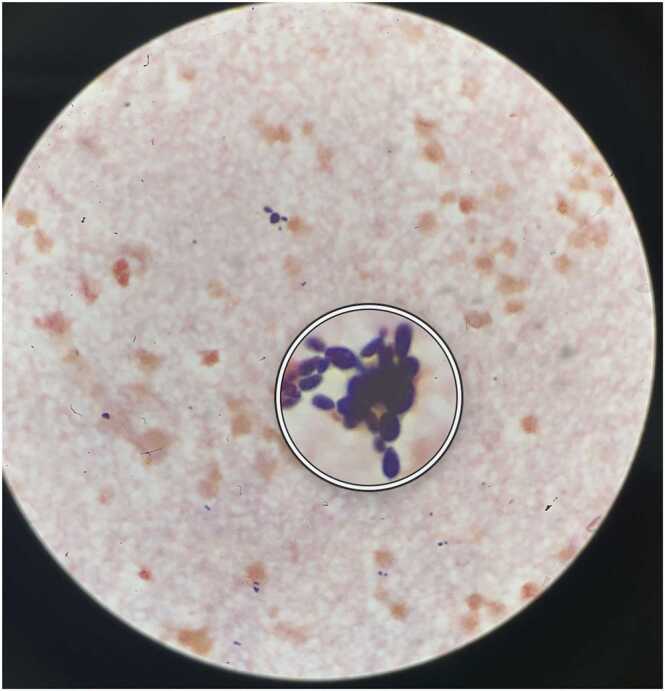
Due to secondary peritonitis and ischemic bowel not amenable to surgery, on HD 14 the patient developed intractable shock refractory to multiple vasopressors, transitioning to comfort care and dying soon thereafter. The ascitic culture from HD 13 was finalized post-mortem as mixed intestinal microbiota with heavy Hyphopichia burtonii (Fig. 3).
Fig. 3.
Peritoneal fluid gram stain on HD 13 showing budding yeast, later identified as Hyphopichia burtonii. The sample was incubated on Sheep Blood Agar, and Hyphopichia burtonii was identified from a BD Phoenix (not pictured). Fig. 4. Proposed pathophysiological mechanism of Spontaneous Bacterial Peritonitis (SBP) in patients with cirrhosis.
Discussion
This case highlights the importance of distinguishing between spontaneous and secondary peritonitis (Table 1). Common signs and symptoms of peritonitis include fever, abdominal tenderness and pain, altered mental status, and hypotension, although it is important to note that SBP can present with hepatic encephalopathy alone. Notably, tense ascites can mask the presence of a rigid abdomen, as was the case with our patient.
Table 1.
Types of Peritonitis in Patients with Cirrhosis.
| Spontaneous Bacterial Peritonitis | Secondary Peritonitis | Spontaneous Fungal Peritonitis | Fungal Ascites | |
|---|---|---|---|---|
| Ascitic Fluid Polymorphonuclear Leukocytes (cells/mm3) | ≥ 250 | ≥ 250 | ≥ 250 | < 250 |
| Ascitic Fluid Microbiologic Culture |
Negative or positive, generally monomicrobial | Negative or positive, usually polymicrobial |
Positive, with fungi present | Positive, with fungi present |
| Clinical Pearls | Prevalent in 7–30% of hospitalized patients with ascites [5] | Surgically treatable source present | Graver prognosis than spontaneous bacterial peritonitis [5], [6] | Candida albicans is the most common fungal isolate [7] |
Spontaneous peritonitis occurs due to translocation or hematogenous spread of bacteria or fungi in the setting of altered intestinal permeability [3] (Fig. 4). SBP is the most common ascitic infection in patients with cirrhosis and is diagnosed when ascitic PMNs are ≥ 250 cells/mm3, regardless of culture data. Prevalence of SBP is 7–30% in hospitalized patients with cirrhosis and ascites and 1.5–3.5% in outpatients with cirrhosis and ascites [5]. Mortality for SBP is high, ranging from 10% to 50% during the first episode and 31–93% following subsequent episodes [5], [8]. Secondary peritonitis differs from spontaneous peritonitis based on the presence of a surgically treatable source of abdominal infection. Secondary peritonitis occurs when gastrointestinal contents leak and directly contaminate the peritoneal cavity and tends to be polymicrobial while spontaneous peritonitis tends to be monomicrobial. Other signs of secondary peritonitis include a poor response to antibiotics, rising ascitic PMN counts on serial paracenteses, and abnormal imaging findings [8]. Runyon’s Criteria can help guide diagnosis, with a 67% sensitivity and 96% specificity for secondary peritonitis (Table 2) [8], [9]. However, the clinical utility of Runyon’s Criteria is limited given its low sensitivity and the infrequent monitoring of peritoneal glucose and LDH levels in everyday practice. Etiologies of secondary peritonitis include bowel obstruction, cholecystitis and perforated ulcers, appendicitis, or diverticulitis. Secondary peritonitis, while less common than spontaneous bacterial peritonitis, carries a 3-fold higher risk of in-hospital death and is nearly 100% fatal without surgical intervention [8].
Fig. 4.
Proposed pathophysiological mechanism of Spontaneous Bacterial Peritonitis (SBP) in patients with cirrhosis.
Table 2.
Runyon’s Criteria for Secondary Peritonitis [9].
| The presence of two of the following three ascitic fluid laboratory criteria have a 67% sensitivity and 96% specificity: |
|---|
| 1. Total protein greater than 1 g/deciliter |
| 2. Glucose less than 50 milligrams/deciliter |
| 3. Lactate dehydrogenase (LDH) greater than the upper limit of normal for serum |
There are little data regarding the prevalence of fungal peritonitis in patients with cirrhosis. Cirrhotic patients are predisposed to fungus growth within the intestinal flora due to baseline immunodeficiency and antibiotic use for SBP prevention [10]. Spontaneous fungal peritonitis is defined as a positive ascitic fungal cultures and ascitic PMNs ≥ 250 cells/mm3. Conversely, fungal ascites is present when ascitic PMNs ≤ 250 cells/mm3 with a positive fungal culture. Candida albicans is the most common peritoneal culture fungal isolate, followed by Candida glabrata and Candida parapsilosis [7]. Fungal peritonitis portends a graver prognosis than bacterial peritonitis, with up to 56% 30-day mortality in cirrhotic patients with Candida peritonitis [6], [7]. Risk factors for fungal peritonitis in cirrhotic patients include ICU admission, parenteral nutrition, vascular or abdominal devices, abdominal surgery, recent antibiotics, or a recent history of GI bleeding [6], [10].
Notably, this patient had polymicrobial peritonitis with Hyphopichia burtonii. While it is unlikely that Hyphopichia burtonii contributed to his death, which we attribute to ischemic bowel, it is interesting and unusual that this fungus was the predominant microbe in his peritoneal cultures. Hyphopichia burtonii has previously been reported in the literature once as a bat pathogen causing cutaneous mycosis and once as a human pathogen [1], [2]. Our patient’s Hyphopichia burtonii isolate was incubated on Sheep Blood Agar and the identification was from BD Phoenix (our Matrix-Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) could not identify it). The initial case was a barbastelle bat that was found emaciated with thickened, ulcerated and sloughing skin. Histologically, fungal spores with fungal masses were observed that were morphologically consistent with Hyphopichia burtonii which was confirmed by polymerase chain reaction (PCR) sequencing [2]. The initial human case of Hyphopichia burtonii was in a patient on peritoneal dialysis. Hyphopichia burtonii was again confirmed using PCR sequencing as it was unable to be identified using MALDI- TOF [1]. In the initial human case report, Hyphopichia burtonii was susceptible to amphotericin B, voriconazole, fluconazole, itraconazole, and caspofungin. Our case adds to the growing body of evidence that Hyphopichia burtonii is a possible human pathogen and clarifies the differences between spontaneous and secondary peritonitis.
Consent
Written informed consent was obtained from the patient’s next of kin for publication of this case report and accompanying images.
Ethical Approval
N/a
Funding
The authors (E.F., A.B., I.V., S.A. and B.K.) have no funding to declare.
CRediT authorship contribution statement
Erica Feldman: Conceptualization, Writing – original draft. Amy Bellinghausen: Writing – review & editing. Irene Vodkin: Writing – review & editing. Shira Abeles: Writing – review & editing. Biren Kamdar: Visualization, Writing – review & editing.
Conflict of interest
The authors (E.F., A.B., I.V., S.A. and B.K.) have no conflicts of interest to declare.
Author contribution
EF wrote the paper. AB, IK , SA and BK took part in patient care, took part in the revisions of the report and contributed to the discussion of the case report and approve the final form of the paper.
Acknowledgements
None.
References
- 1.Chamroensakchai T., Kanjanabuch T., Saikong W., et al. The first human report of Hyphopichia burtonii, initially misdiagnosed as sterile peritonitis in a patient on peritoneal dialysis. Med Mycol Case Rep. 2021;33:26–29. doi: 10.1016/j.mmcr.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson V.R., Borman A.M., Fox R.I., et al. Cutaneous mycosis in a Barbastelle bat (Barbastella barbastellus) caused by Hyphopichia burtonii. J Vet Diagn Investig. 2013;25:551–554. doi: 10.1177/1040638713493780. [DOI] [PubMed] [Google Scholar]
- 3.Biggins S.W., Angeli P., Garcia-Tsao G., et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–1048. doi: 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- 4.Riley R., Haridas S., Wolfe K.H., et al. Comparative genomics of biotechnologically important yeasts. Proc Natl Acad Sci USA. 2016;113:9882–9887. doi: 10.1073/pnas.1603941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shizuma T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: a literature review. World J Hepatol. 2018;10:254–266. doi: 10.4254/wjh.v10.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tariq T., Irfan F.B., Farishta M., et al. Spontaneous fungal peritonitis: Micro-organisms, management and mortality in liver cirrhosis-A systematic review. World J Hepatol. 2019;11:596–606. doi: 10.4254/wjh.v11.i7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassetti M., Peghin M., Carnelutti A., et al. Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: a multicenter study. Intensive Care Med. 2017;43:509–518. doi: 10.1007/s00134-017-4717-0. [DOI] [PubMed] [Google Scholar]
- 8.Soriano G., Castellote J., Alvarez C., et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol. 2010;52:39–44. doi: 10.1016/j.jhep.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Akriviadis E.A., Runyon B.A. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127–133. doi: 10.1016/0016-5085(90)91300-u. [DOI] [PubMed] [Google Scholar]
- 10.Li B., Yang C., Qian Z., et al. Spontaneous fungal ascites infection in patients with cirrhosis: An analysis of 10 cases. Infect Dis Ther. 2021;10:1033–1043. doi: 10.1007/s40121-021-00422-w. [DOI] [PMC free article] [PubMed] [Google Scholar]