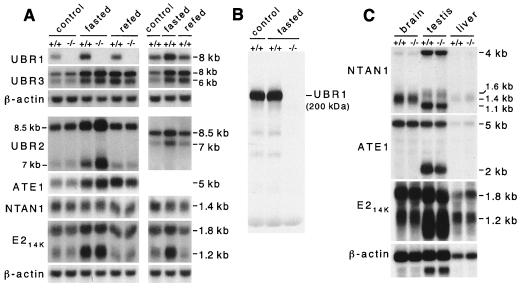
FIG. 5.
Expression analysis of mouse UBR1 and functionally related genes in +/+ and UBR1−/− mice. (A) Northern analysis of skeletal muscle mRNAs encoding UBR1, other components of the N-end rule pathway, and the UBR1 homologs UBR2 and UBR3. RNA was isolated from skeletal muscle of control, 48-h fasted, or 24-h refed +/+ and UBR1−/− mice. See Materials and Methods and the legend to Fig. 4D for the fasting and refeeding protocols. Northern blots were hybridized with 32P-labeled cDNA probes specific for the following genes: UBR1 (nucleotides 555 to 888, accession no. AF061555; this probe was specific for the deleted region in the UBR1− allele); UBR2 (a 0.3-kb probe encompassing the region homologous to the one deleted in the UBR1−/− allele; Y. T. Kwon and A. Varshavsky, unpublished data); UBR3 (a 0.4-kb probe; Y. T. Kwon and A. Varshavsky, unpublished data); ATE1 (nucleotides 638 to 1734, accession no. AF079098); NTAN1 (nucleotides 34 to 900, accession no. U57692); mHR6B (E214K) (nucleotides 115 to 569, accession no. U57690); and β-actin. (B) The level of UBR1 protein in skeletal muscle is not significantly altered upon fasting. Total extracts (∼70 μg per lane) from skeletal muscles of normally fed or 48-h fasted +/+ and UBR1−/− mice were analyzed by immunoblotting, using affinity-purified antibody (38) against the N-terminal ∼35-kDa fragment of mouse UBR1. (C) Northern analysis of ATE1, NTAN1, and mHR6B (E214K) expression in the brain, liver, and testis of +/+ and UBR1−/− mice by using DNA probes described in the legend to panel A.