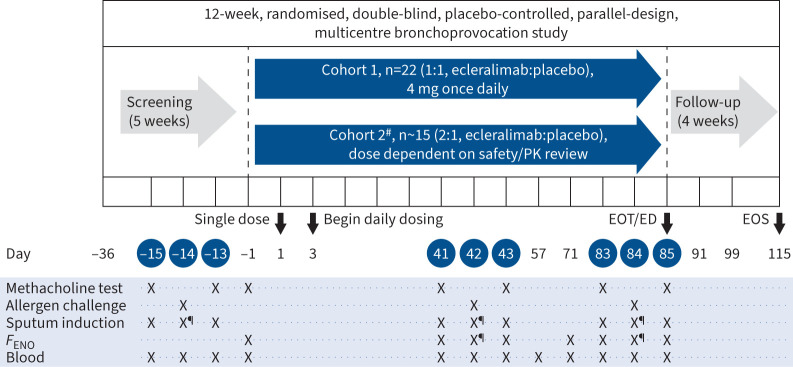
FIGURE 1.
Study design. The efficacy end-points were evaluated on days 42 and 84. #: efficacy interim analysis planned in the middle of Cohort 2 (∼15 subjects enrolled) with the potential to adjust the dose for an additional ∼18 subjects; ¶: 7 h post-challenge. PK: pharmacokinetics; EOT: end of treatment; ED: early discontinuation; EOS: end of study; FENO: fractional exhaled nitric oxide.