Fig. 4. Design of protein function with ProteinMPNN.
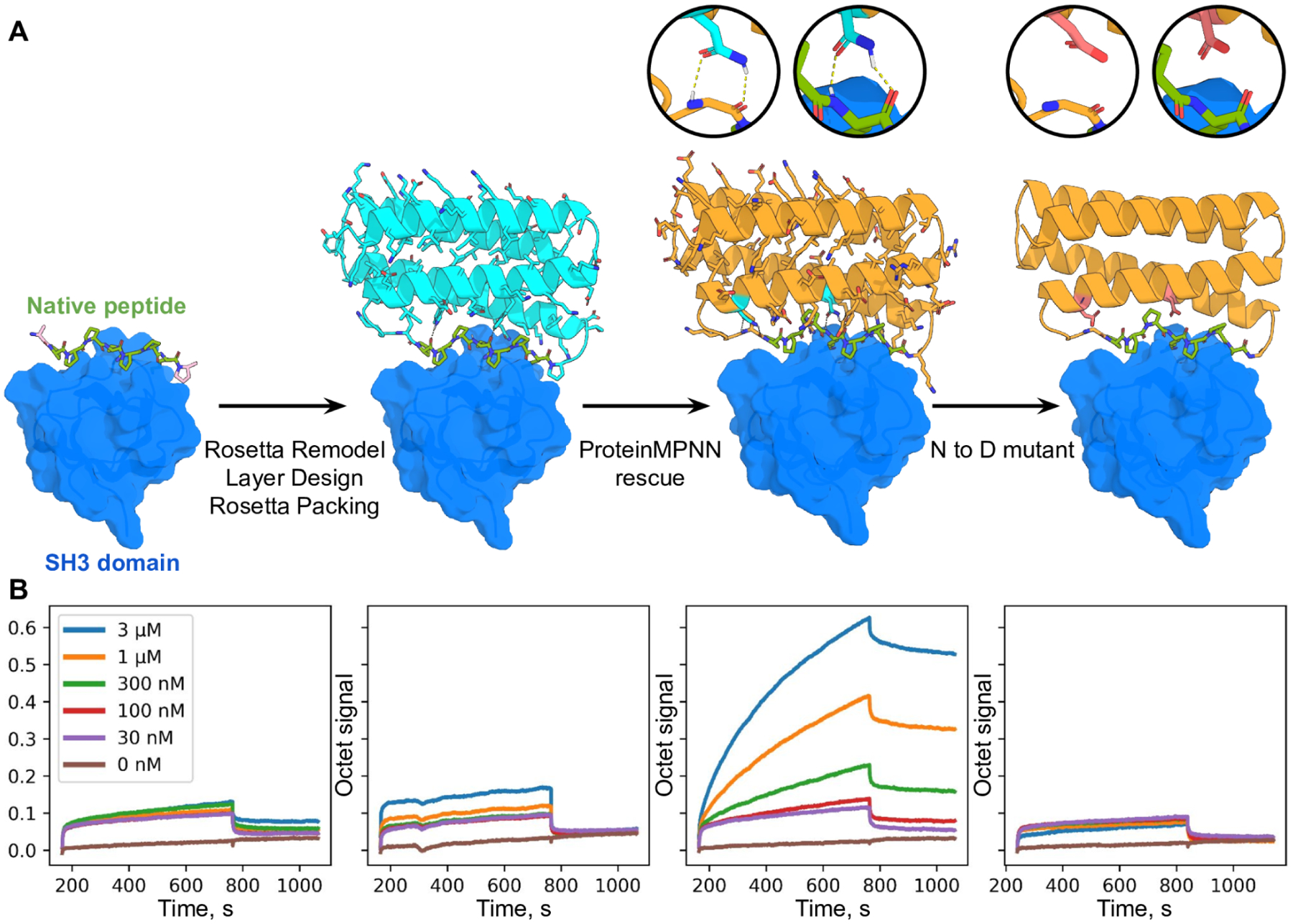
(A) Design scheme. First panel; structure (PDB 2W0Z) of the peptide APPPRPPKP bound to the human Grb2 C-term SH3 domain (peptide is in green, target in surface and colored blue). Second panel: helical bundle scaffolds were docked to the exposed face of the peptide using RIFDOCK (19), and Rosetta remodel was used to build loops connecting the peptide to the scaffolds. Rosetta sequence design with layer design task operations was used to optimize the sequence of the fusion (Cyan) for stability, rigidity of the peptide-helical bundle interface, and binding affinity for the Grb2 SH3 domain. Third panel; ProteinMPNN redesign (orange) of the designed binder sequence; hydrogen bonds involving asparagine sidechains between the peptide and base scaffold are shown in green and in the inset. Fourth panel; Mutation of the two asparagines to aspartates to disrupt the scaffolding of the target peptide. (B) Experimental characterization of binding using biolayer interferometry. Biotinylated C-term SH3 domain from human Grb2 was loaded onto Streptavidin (SA) Biosensors, which were then immersed in solutions containing varying concentrations of the target peptide (left) of the designs (right panels), and then transferred to buffer lacking added protein for dissociation measurements. The MPNN design (3rd panel from the left) has much greater binding signal than the original Rosetta design (2nd panel from the left); this is greatly reduced by the asparagine to aspartate mutations (last panel).
