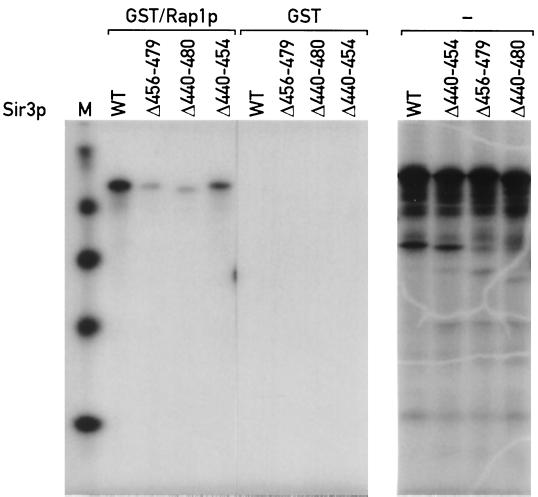
FIG. 2.
Sir3p mutants with a deletion of amino acids 456 to 479 are defective in binding to GST/Rap1p(562–827) in vitro. See Materials and Methods for details of the binding assay. Right, in vitro-produced labeled wild-type (WT) and mutant proteins (from a separate gel) before addition to GST/Rap1p(562–827) or GST-agarose beads. Left, material bound to the GST/Rap1p beads in each lane [Sir3p(1–978), Sir3p(Δ456–479), Sir3p(440–480), and Sir3p(440–454)] has the same mobility as the primary high-molecular-weight translation product in the right panel (note that the order of lanes in the two gels is not the same). Lane M, size standards.