FIGURE.
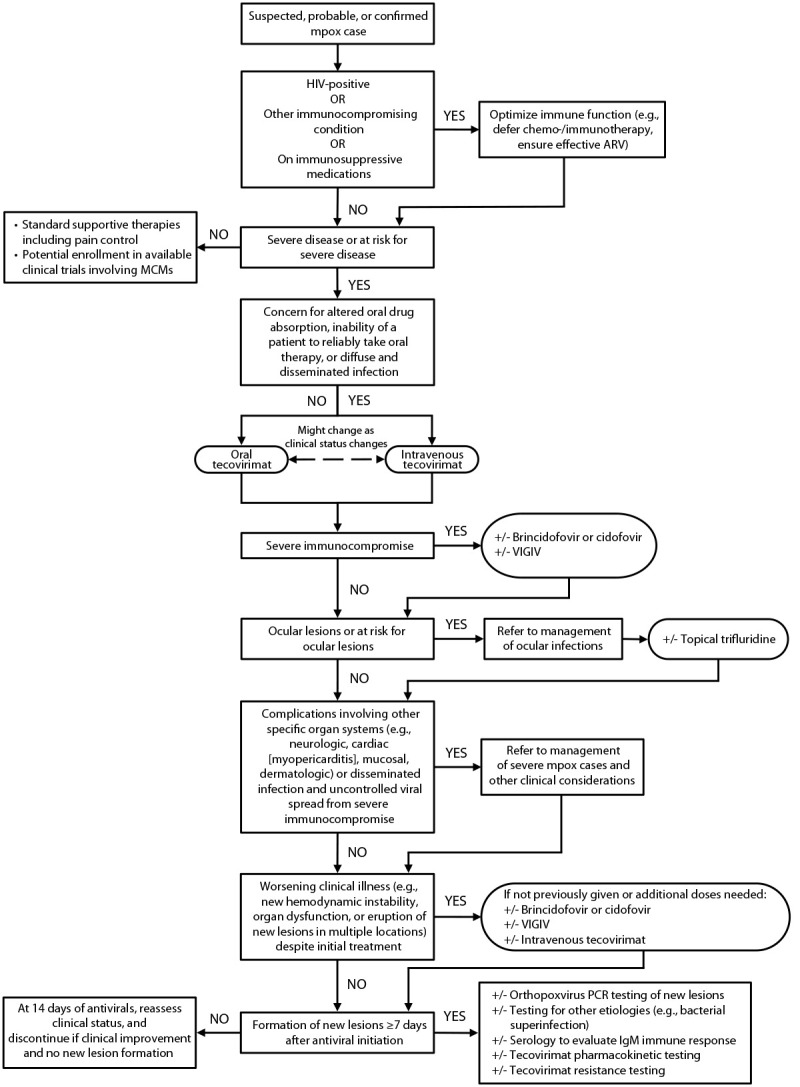
Approach to treatment*,†,§ of patients with severe¶ or at risk** for severe manifestations of mpox†† — United States, February 2023§§
Abbreviations: ARV = antiretroviral medications; GI = gastrointestinal; IgM = immunoglobulin M; MCM = medical countermeasure; PCR = polymerase chain reaction; VIGIV = vaccinia immune globulin intravenous.
* Treatment includes MCMs (i.e., tecovirimat, brincidofovir, cidofovir, VIGIV, and trifluridine) and supportive therapies, including pain management. https://www.cdc.gov/poxvirus/monkeypox/clinicians/pain-management.html
† Most immunocompetent patients should display signs of clinical improvement within 4 days of antiviral initiation (i.e., tecovirimat, brincidofovir, cidofovir, and trifluridine). Tecovirimat is expected to reach steady state concentrations by day 6 of dosing in healthy volunteers; therefore, worsening clinical illness after 7 days of treatment in patients with severe illness could prompt additional evaluations.
§ Concern for altered drug absorption includes the inability to tolerate or take oral therapy (e.g., nothing by mouth), or possibility that the oral drug absorption might be altered because of inability to consume a high-fat meal, severity of symptoms (e.g., systemic illness), comorbidities (e.g., history of gastric bypass or underlying GI disease), or other factors that might alter oral drug absorption.
¶ Hemorrhagic disease, a large number of confluent or necrotic lesions, severe lymphadenopathy that is necrotizing or obstructing (e.g., of the upper airway causing airway compromise or of the GI tract necessitating parenteral feeding), edema that is obstructing (e.g., of the lower GI tract), extradermatologic manifestations (e.g., pulmonary nodules, encephalitis, myopericarditis, or ocular infections), and sepsis. Detailed characteristics of severe disease are available at https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#anchor_1655488137245.
** Persons with underlying medical conditions (e.g., severe or moderate immunocompromise [https://www.cdc.gov/poxvirus/monkeypox/clinicians/people-with-HIV.html]); bacterial superinfections; or complications, including strictures, edema, and infections of the penile foreskin, vulva, urethral meatus, or anorectum, which could require procedural intervention (e.g., urethral catheterization, colostomy, or surgical debridement). This also includes those with or at risk for ocular lesions (i.e., presence of eyelid lesions, facial lesions near the eyes, or finger or hand lesions in patients unable to avoid touching their eyes [for whom autoinoculation is a concern]). Detailed characteristics of persons at risk for severe disease are available at https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html#anchor_1655488137245.
†† https://www.cdc.gov/poxvirus/monkeypox/clinicians/case-definition.html
§§ This figure is a comprehensive synthesis of heterogeneous evidence and is intended to foster strategic decision-making rather than serve as a prescriptive treatment guideline.
