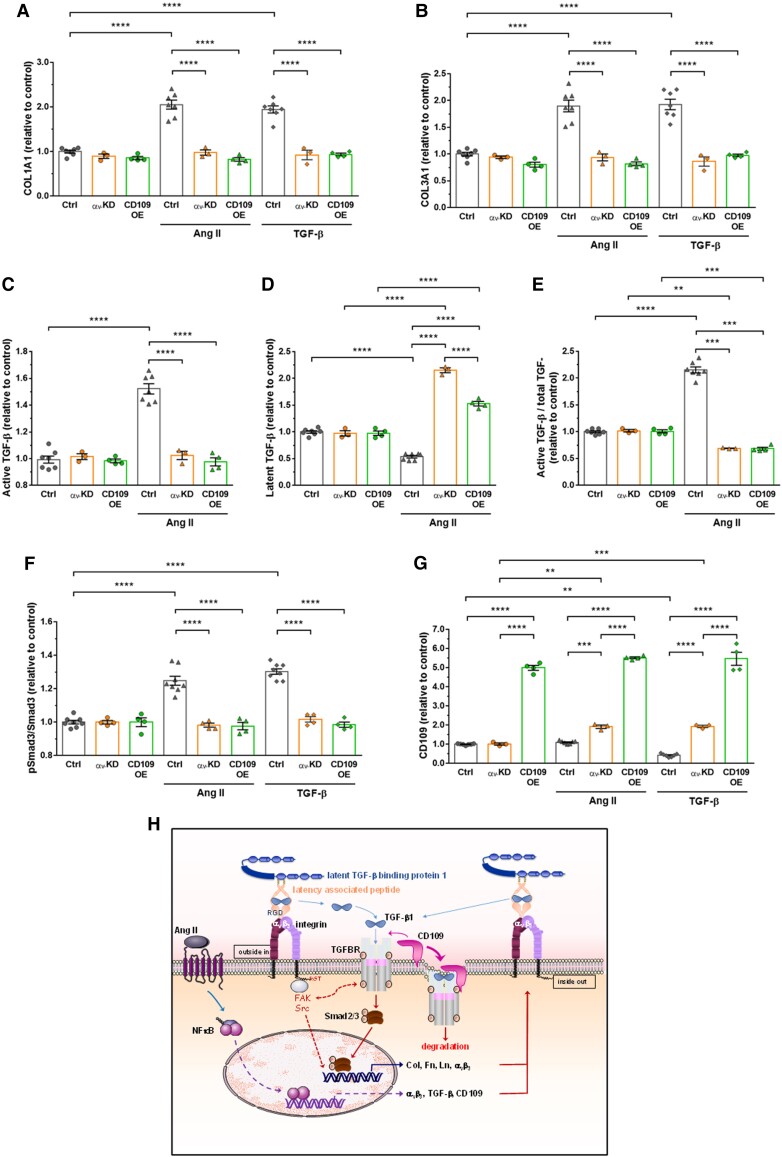
Figure 6.
Knockdown of αv integrin subunit or overexpression of CD109 inhibited human aortic VSMC responses to Ang II or TGF-β1. (A–B) Quantification of COL1A1 and COL3A1 by ELISA in lysates of VSMCs transfected with the control siRNA or empty vector (EV), a siRNA against αv (αv-KD) or an adenovirus expressing mouse CD109 (CD109-OE) treated for 48 h with Ang II or TGF-β1. (C–E) Quantification active and latent TGF-β by ELISA in lysates of EV or αv-KD VSMCs treated for 48 h with Ang II. (F–G) Quantification of phosphorylated Smad3 and CD109 in EV, αv-KD, or CD109-OE VSMCs treated for 48 h with Ang II or TGF-β1. All data are presented as mean ± SEM (n = 3–8 per group). Two-way ANOVA followed by Tukey’s multiple comparisons test was used to analyse multiple group comparisons. ***P < 0.001, **** P < 0.0001. (H) Schematic model of the mechanism underlying αv integrin and TGF-β/CD109 signalling cross-talk in Ang II-induced vascular fibrosis. The binding of αv integrins to the RGD motif present in LAP leads to the liberation/activation of the TGF-β homodimer from its latent complex. After activation, the TGF-β homodimer binds to TGF-β receptors, thereby initiating TGF-β-Smad2/3 signalling, which increases Col, Fn, Ln, αv integrins, TGF-β, and CD109 expressions. These newly formed integrins will reactivate TGF-β from its latent complex, reinforcing the role of TGF-β on collagen synthesis. Membrane-anchored CD109 enhances binding of TGF-β to TGF-β receptors, which in turn promotes internalization of the complex TGF-β/TGF-β receptor/CD109 and subsequent proteasomal degradation of TGF-β receptor. Activation of TGF-β in response to Ang II seems independent of circumferential wall stress changes (see text for details).