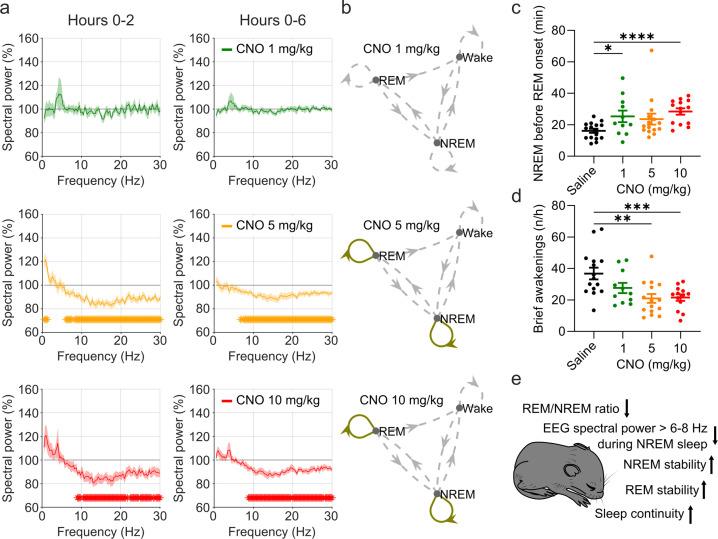
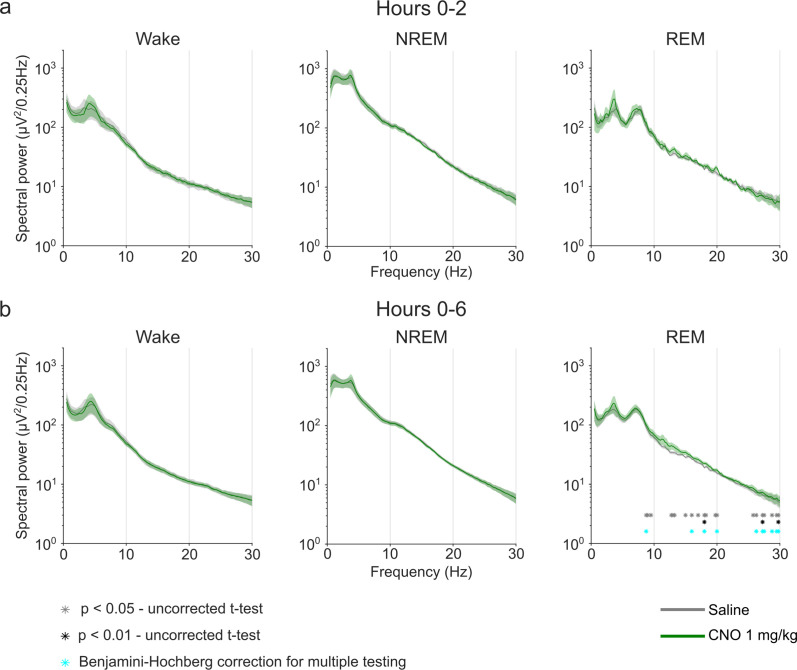
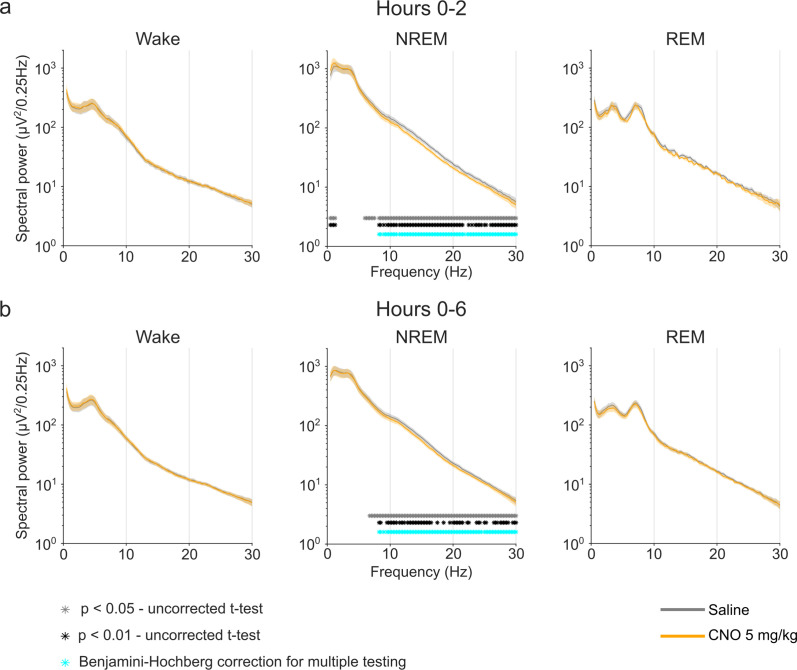
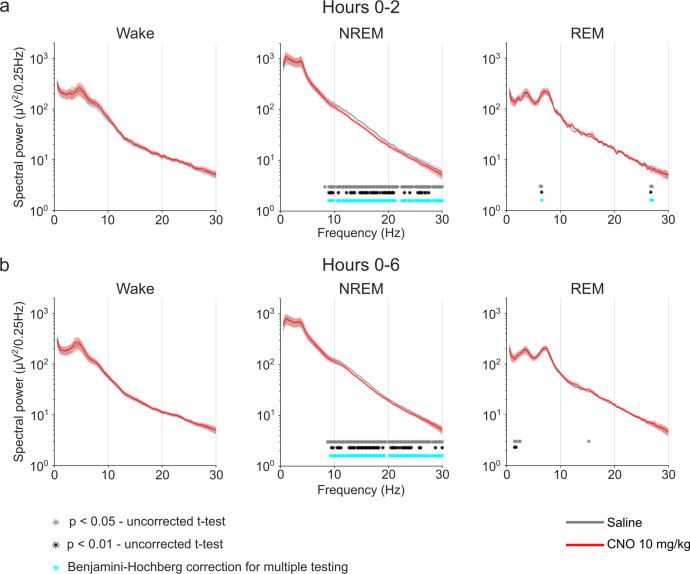
Figure 3. EEG spectral changes, increased sleep state stability, and sleep continuity following CNO injections.
(a) Frontal EEG spectra during NREM sleep following CNO injections relative to saline injections for the acute (first 2 hr, left column) and prolonged (6 hr, right column) observation period. Note the sustained reduction of power in frequency bands >6–8 Hz in the 5 and 10 mg/kg CNO conditions. (b) Transitions between vigilance states in the 6 hr period following saline and CNO injections. Note the increased stability of REM and NREM sleep for the 5 mg/kg CNO (REM>REM: p=0.0192, Cohen’s d=0.73681; NREM>NREM: p=0.0132, Cohen’s d=0.71052) and 10 mg/kg CNO (REM>REM: p=0.0492, Cohen’s d=0.65815; NREM>NREM: p=0.0214, Cohen’s d=0.77396) condition. Solid olive lines indicate significantly increased transitions/continuations of vigilance states in the respective CNO condition compared to the saline condition, dashed grey lines indicate all possible vigilance state transitions/continuations. (c) Cumulative amount of NREM sleep before the first occurrence of REM sleep. (d) Frequency of brief awakenings (4–16 s) per hour of sleep for the first 2 hr after injections. (e) summary of effects of 5 and 10 mg/kg CNO on sleep in DREADD-free mice. n=10 for saline, n=6 for 1 mg/kg, n=10 for 5 mg/kg, n=8 for 10 mg/kg for spectral analysis. n=16 for saline, n=11 for 1 mg/kg, n=15 for 5 mg/kg, n=14 for 10 mg/kg for vigilance state analysis. n=15 for saline, n=11 for 1 mg/kg, n=15 for 5 mg/kg, n=13 for 10 mg/kg for analysis of brief awakenings. Asterisks in panels c and d indicate post hoc comparisons with significant differences (*p<0.05, **p<0.01, ***p<0.001,, ****p<0.001). Asterisks in panel a indicate frequency bins with significant differences in post hoc comparisons using uncorrected paired t-tests (p<0.05) following a significant interaction effect between ‘frequency’ and ‘condition’ in two-way ANOVAs. Data in panel a are presented as the mean ± s.e.m. (shaded areas). ANOVA: analysis of variance. CNO: clozapine-N-oxide. EEG: electroencephalogram. NREM: non-rapid eye movement sleep.