Figure 4. U90926 RNA localizes to the cytoplasm, associates with ribosomes, and contains an ORF that encodes a secreted protein.
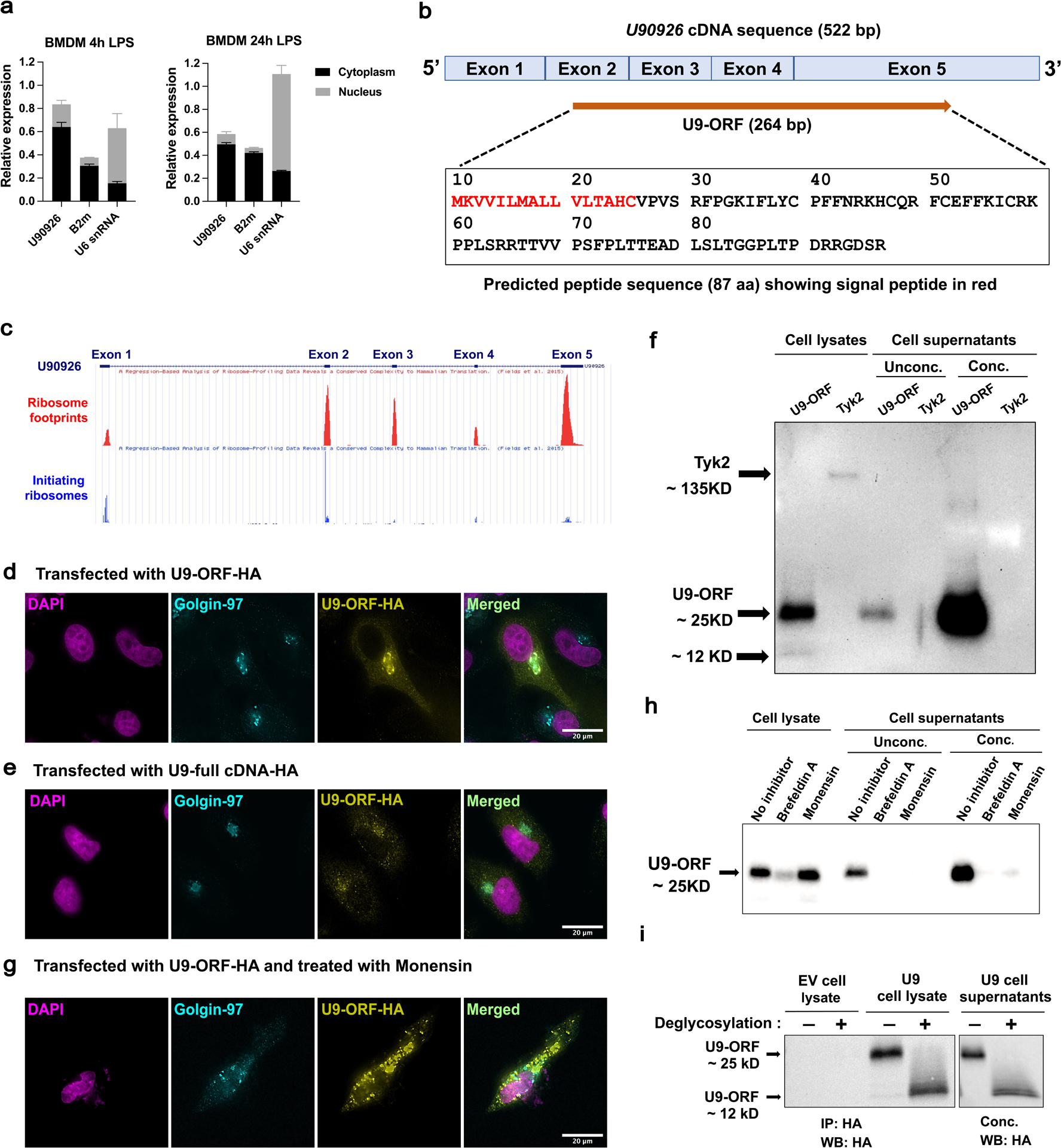
(a) WT BMDM were stimulated for 4h and 24h with LPS (100ng/ml), followed by isolation of nuclear and cytoplasmic fractions and RTq-PCR analysis of U90926, B2m (cytoplasmic control), and U6 SnRNA (nuclear control) expression (each normalized to unfractionated input as equal to 1). (b) A schematic diagram of U90926 cDNA sequence (522 bp) containing the 264 bp ORF spanning the exons is shown. Predicted amino acid sequences of the U9-ORF peptide (87 aa) and the signal peptide identified by SignalP software shown in red. (c) GWiPS was used to analyze of Ribo-seq data from LPS stimulated BMDC(46) at the U90926 locus. Global ribosome footprints or initiating ribosomes footprint data (in the presence of harringtonine) are shown, as indicated. (d, e) HeLa cells were transfected with U9-ORF-HA (d) and U9-full-lengthcDNA-HA (e) plasmids, followed by immunostaining for HA (yellow) and Golgin-97 (cyan; Golgi marker), and DAPI nuclear staining (magenta). (f) HeLa cells were transfected with U9-ORF-HA and TYK2-HA plasmids for 48 hours, followed by immunoblot analysis. Supernatants were loaded directly or concentrated using StrataClean resin, as indicated (Unconc. or Conc., respectively). (g) HeLa cells were transfected with U9-ORF-HA for total 48 hours, where protein transport inhibitor, monensin was added at 24 hours, and then cells were subjected to immunostaining at 48 hours of transfection, as above. (h) HeLa cells were transfected with U9-ORF-HA for a total of 48 hours, in which protein transport inhibitors, brefeldin A and monensin were added at 24 hours, and sample collection was done at 48 hours of transfection, followed by immunoblot analysis. Supernatants were analyzed as described above. (i) HeLa cells were transfected with empty vector control and U9-ORF-HA plasmid for 48 hours. Cell lysates were immunoprecipitated using an anti-HA antibody and supernatants were concentrated using StrataClean resin, followed by deglycosylation under denaturing conditions overnight at 37°C and analyzed by immunoblot.
