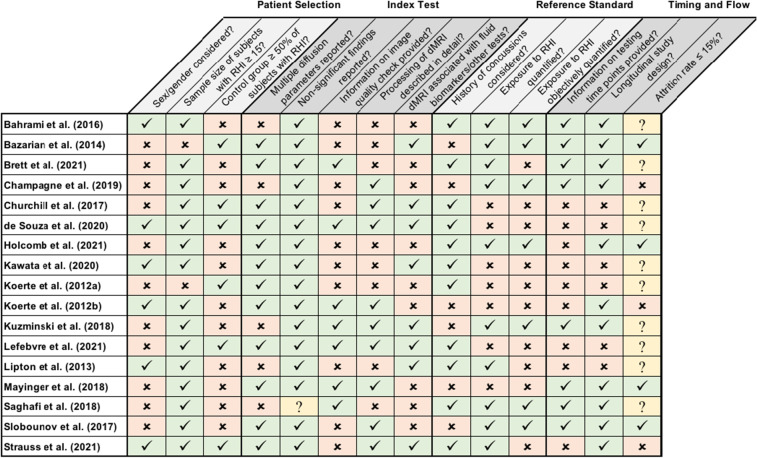
Table 3.
Summary of risk of bias assessment using QUADAS-2 based rating of methodological study quality
The following items were selected for reviewing the four domains. A check mark on the green background indicates that the answer to the question was “Yes”, while an X on the peach-colored background indicates that the answer to the question was “No”. A question mark on yellow background indicates that the answer was “Not applicable/Unclear”. A) Patient Selection: 1. Have sex differences been investigated OR were statistical analyses controlled for sex OR was sex reported in the discussion/limitations section of the manuscript? 2. Was the included number of exposed athletes sufficiently large (n ≥ 15)? 3. Has a control group been included AND was the percentage of controls ≥ 50% of those in the RHI exposed athlete sample? B) Index Test: 1. Were two or more dMRI measures (FA, MD, etc.) reported? 2. Have non-significant findings also been reported in the results? 3. Was a quality check of dMRI data described in detail and were measures taken appropriate for ensuring sufficient quality of data? (i.e., visual inspection of raw data, use of software for quality check of raw and processed data). 4. Was processing of dMRI data described in sufficient detail to ensure reproducibility and was software used appropriate? 5. Have diffusion measures been associated with fluid biomarkers or other tests? C) Reference Standard: 1. Were individuals with a history of concussion excluded OR was history of concussion considered in the statistical analyses? 2. Has exposure to RHI been quantified? 3. Has exposure to RHI been objectively quantified (e.g., counting RHI, or using sensors)? D) Flow and Timing: 1. Was testing time point specified and was a rationale of choice of testing time point reported? (i.e., testing time before/within/after a specific sports season and related to purpose of the study i.e., pre-postseason comparison) 2. Was attrition rate of participants ≤ 15%? 3. Was it a longitudinal study design?