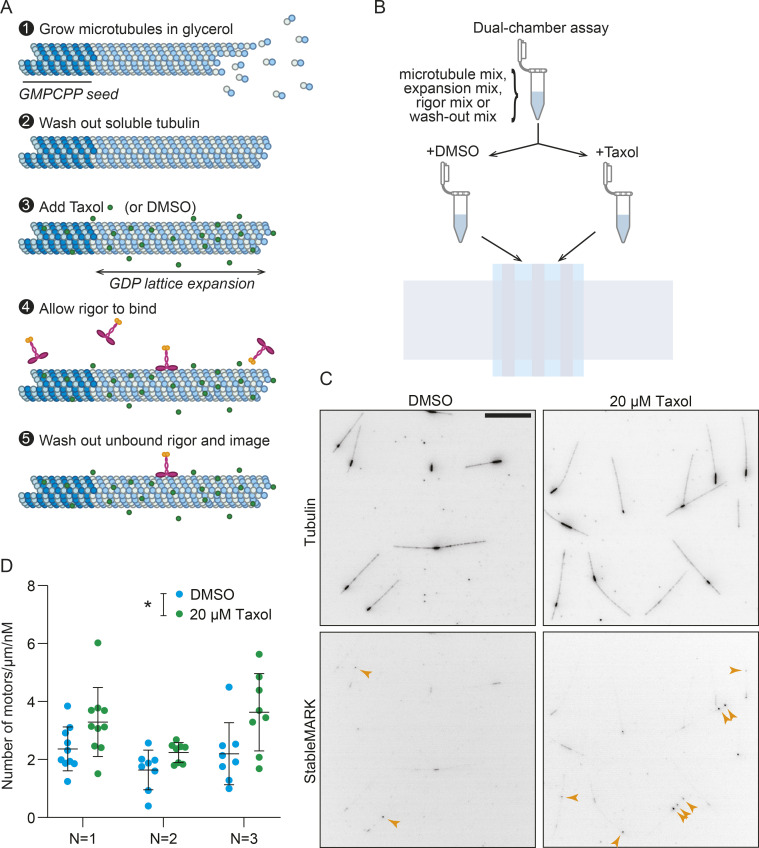
Figure S2.
StableMARK shows a preference for expanded lattices in vitro. Related to Fig. 2. (A) Schematic showing the assay setup. All steps were done in high glycerol buffer. MTs were polymerized from immobilized GMPCPP-stabilized seeds for 15 min (1). After washing out soluble tubulin (2), 20 µM Taxol was added and allowed to incubate to expand the MT lattice (3), or DMSO was added as a control. Subsequently, 15.2 pM StableMARK was added and allowed to bind for 90 seconds (4), before washing out unbound rigor and imaging (5). (B) Assays were performed paired, with buffers for all steps prepared together and then split into two equal parts for the addition of Taxol or DMSO. These buffers were introduced into two flow cells on the same coverslip, treated, and imaged concomitantly. (C) Representative images showing the MTs (top; dark: GMPCPP seed, light: GDP lattice) and StableMARK (bottom; sum projection). Arrowheads indicate rigor bound to GDP segments. (D) Quantification thereof with the number of bound molecules counted per µm of GDP lattice traced per nM of StableMARK added. For each independent experiment, there is an increase in the average density of motors on the GDP lattice in the Taxol-treated condition, suggesting that StableMARK shows a preference for expanded lattices. n = 8–10 fields of view for N = 3 independent experiments. The mean ± SD is shown for each experiment. *P < 0.05 (ratio paired t test of the means; normality assumed, not formally tested). Note that the off-rate of StableMARK is ∼0 in all conditions tested in vitro and this could limit the difference observed. Scale bar, 10 µm (C).