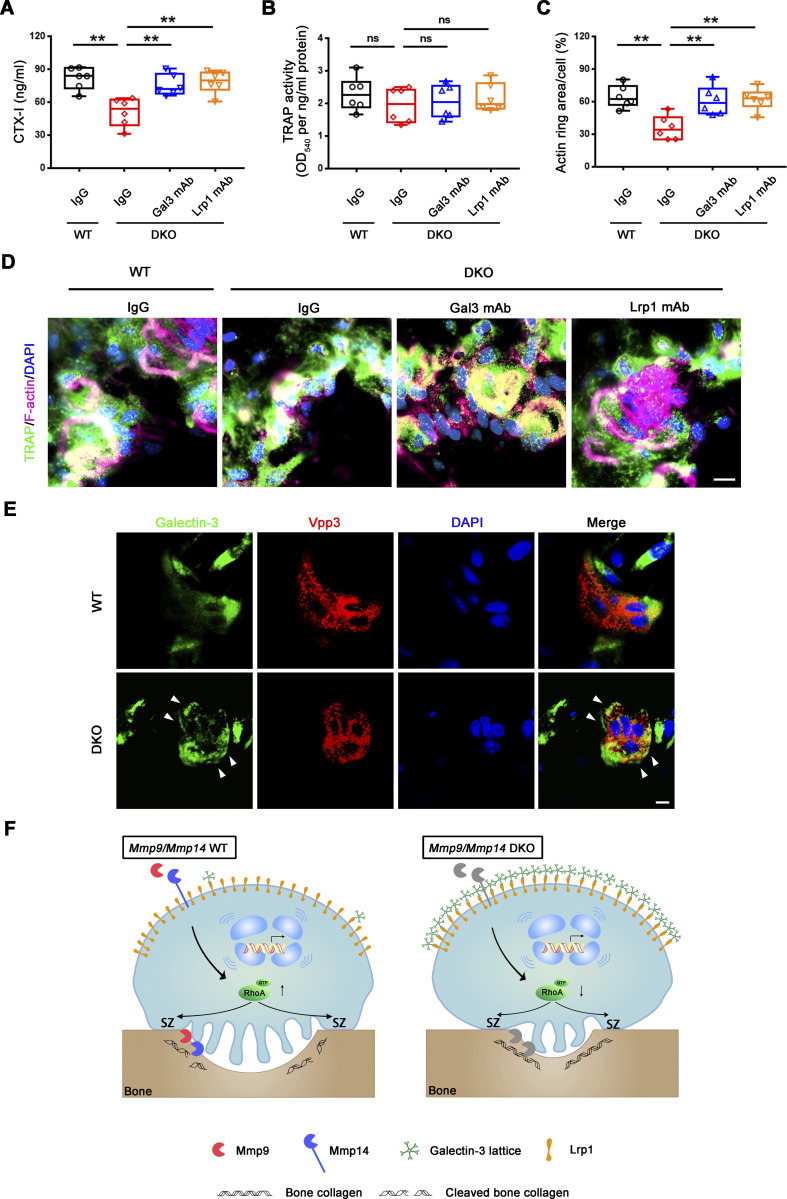
Figure 10.
Mmp9/Mmp14–galectin-3–Lrp1 axis controls osteoclast activity in a calvaria explant model. (A and B) Calvaria isolated from wild-type and DKO mice were cultured in the presence or absence of either the galectin-3 or Lrp1 function-blocking mAbs (25 μg/ml) for 5 d at 37°C, and supernatants or whole cell lysates collected for CTX-I ELISA (A) and TRAP activity (B), respectively. Data are presented as mean ± SEM (n = 6 biological replicates). (C and D) Actin ring area per cell (C) and phalloidin staining (magenta) with TRAP (green) immunofluorescence with an anti-TRAP polyclonal antibody (sc-30833; Santa Cruz; D) are shown of wild-type and DKO calvaria explants cultured in the presence or absence of either galectin-3 or Lrp1 function-blocking mAbs (25 μg/ml) for 5 d at 37°C. Scale bar, 10 μm. Data are presented as mean ± SEM (n = 6 biological replicates). **P < 0.01. Statistical significance was assessed using one-way ANOVA with Bonferroni correction. (E) Galectin-3 (green) and Vpp3 (red) immunofluorescence stained with an anti–galectin-3 monoclonal antibody (#125401; Biolegend; clone M3/38) and an anti-Vpp3 polyclonal antibody (Abcam, ab200839) of wild-type and DKO calvarial organ. White arrowhead, galectin-3 lattice. Scale bar, 10 μm. Results are representative of three independent experiments. (F) Schematic depicting dual roles for MMP9 and MMP14 in osteoclast-mediated bone resorption by both modulating the galectin-3/Lrp1-dependent control of RhoA-GTPase activity, but also serving as direct-acting bone type I collagenolysis (Zhu et al., 2020). Galectin-3 is depicted as a pentamer only as an example of a potential multimer complex.