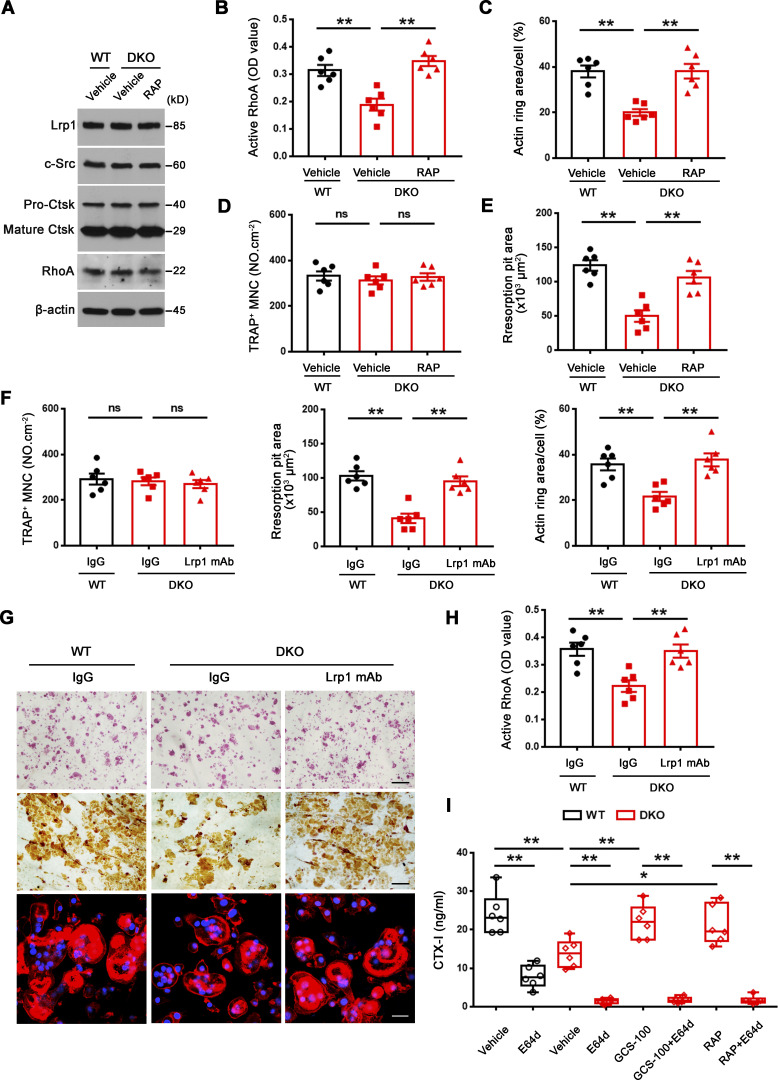
Figure 8.
Inhibition of Lrp1 function reverses defects in RhoA activation, sealing zone formation and bone resorption in DKO osteoclasts. (A) c-Src, Ctsk, and RhoA expression in wild-type osteoclasts treated with vehicle, and DKO osteoclasts treated with vehicle or 50 nM RAP as assessed by Western blot. Results are representative of three independent experiments. (B) Wild-type osteoclasts were treated with vehicle, and DKO osteoclasts were treated with vehicle or 50 nM RAP for 2 h at 37°C, and RhoA activity determined upon activation with 20 ng/ml M-CSF and 30 ng/ml RANKL for 15 min. Data are presented as mean ± SEM (n = 6 biological replicates). (C–E) Phalloidin staining of wild-type pre-osteoclasts treated with vehicle, and DKO pre-osteoclasts treated with vehicle or 50 nM RAP cultured on bone slices for 3 d at 37°C, and actin ring area per cell quantified (C). Cells were stained for TRAP activity and the number of TRAP+ MNCs quantified (D). Osteoclasts were removed and resorption pits visualized by WGA-DAB staining and resorption pit area quantified (E). Data are presented as mean ± SEM (n = 6 biological replicates). (F and G) TRAP (red), WGA-DAB, and phalloidin staining (red) of wild-type pre-osteoclasts cultured atop bone slices treated with control IgG, and DKO pre-osteoclasts treated with control IgG or Lrp1 function-blocking mAb (25 μg/ml) for 3 d at 37°C (G), and the number of TRAP+ MNCs, actin ring area per cell, and resorption pit area quantified (F). Scale bar, upper and middle 100 μm, lower 20 μm. Data are presented as mean ± SEM (n = 6 biological replicates). (H) Wild-type osteoclasts were treated with control IgG, and DKO osteoclasts were treated with control IgG or Lrp1 function-blocking mAbs at 25 μg/ml for 2 h, and RhoA activity determined upon activation with 20 ng/ml M-CSF and 30 ng/ml RANKL for 15 min. Data are presented as mean ± SEM (n = 6 biological replicates). (I) Pre-osteoclasts differentiated from wild-type or DKO BMDMs were cultured atop cortical bone slices with or without 20 μM E64d, 20 μg/ml GCS-100, or 50 nM RAP for 3 d at 37°C, and supernatants collected for CTX-I ELISA. Data are presented as mean ± SEM (n = 6 biological replicates). *P < 0.05, **P < 0.01. Statistical significance was assessed using one-way ANOVA (B–F, H) and two-way ANOVA (I) with Bonferroni correction. Source data are available for this figure: SourceData F8.