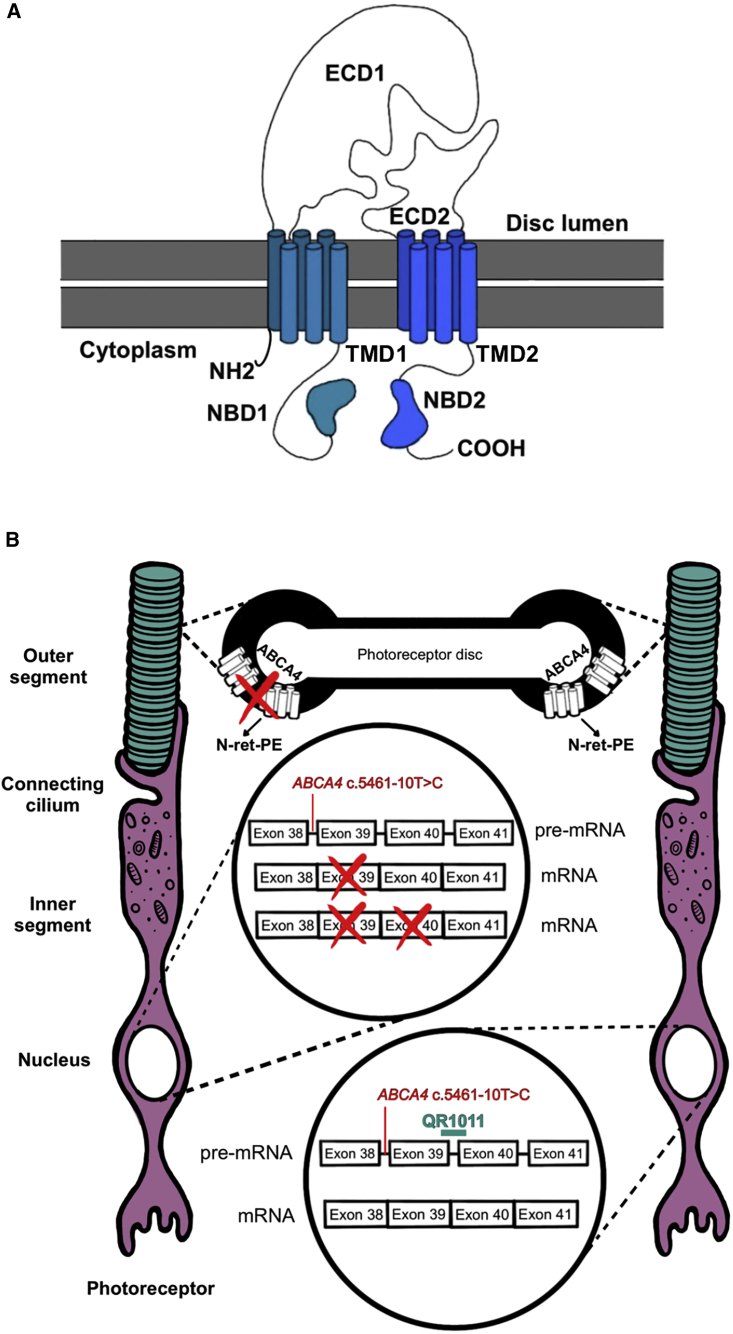
Figure 1.
Structure of ABCA4 and the splicing modulating effect of QR-1011
(A) The complex structure of the ABCA4 protein involves two transmembrane domains (TMD1 and TMD2), each with six transmembrane helices. In addition, the protein structure displays two glycosylated extra cytoplasmatic domains (ECD1 and ECD2) and two nucleotide-binding domains (NBD1 and NBD2) where ATP hydrolysis takes place. (B) In presence of the frameshift variant ABCA4 c.5461-10T>C, generated transcripts lack either the exon 39 or exons 39 and 40; this splicing defect hampers the production of functional ABCA4 protein and toxic retinoid products (N-retinylidene-phosphatidylethanolamine [PE]) cannot be removed from the photoreceptor’s outer segments, leading to the accumulation of A2E and lipofuscin granules, key pathogenic features for STGD1. The splicing modulating activity of QR-1011 is designed to restore wild-type splicing and include exons 39 and 40 in ABCA4.