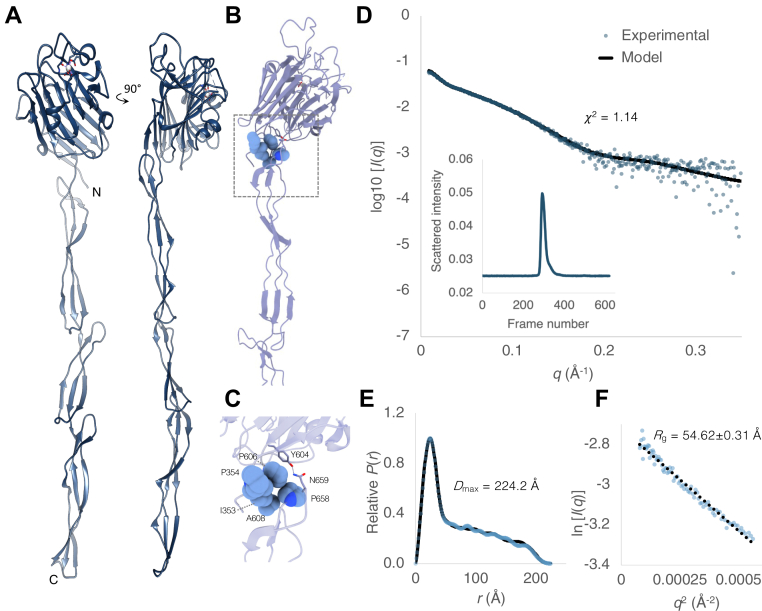
Figure 5.
TheX-ray crystal structure and SAXS analysis of a contiguous adhesin domain and rod-like B region are consistent with exposure of the Aap ligand binding site at the cell surface. (A) Crystal structure of Aap351–813 showing rod-like B region and lectin-like A region with N and C termini annotated. A disordered loop is illustrated as a dashed line. B residues forming a van der Waals interface between the lectin domain and B repeat region G51 are illustrated as spheres, and hydrogen bonding side chains are shown as cylinders. These residues are annotated in a magnified image in (C). D, fit of the Aap351–813 model to experimental scattering data. The data are accounted for by a single model of Aap351–813. Experimental data (blue) and calculated (black) scattering curves are displayed to a maximal momentum transfer of q = 0.35 Å, with fit value (χ2) annotated. D (inset): size exclusion chromatography trace of Aap351–813 sample injection. E, normalized pair-distance distribution function (P(r)) with calculated maximum intraparticle diameter (Dmax) displayed. Experimental data (blue) and calculated (black) curves are displayed. Real space Rg = 64.55 ± 0.9 Å; I(0) = 0.069. F, guinier region; linearity confirms monodispersity (q∗Rg range 0.48–1.29; Rg = 54.62 ± 0.31 Å; I(0) = 0.065). The dotted line shows linear regression with an R2 value of 0.9772.