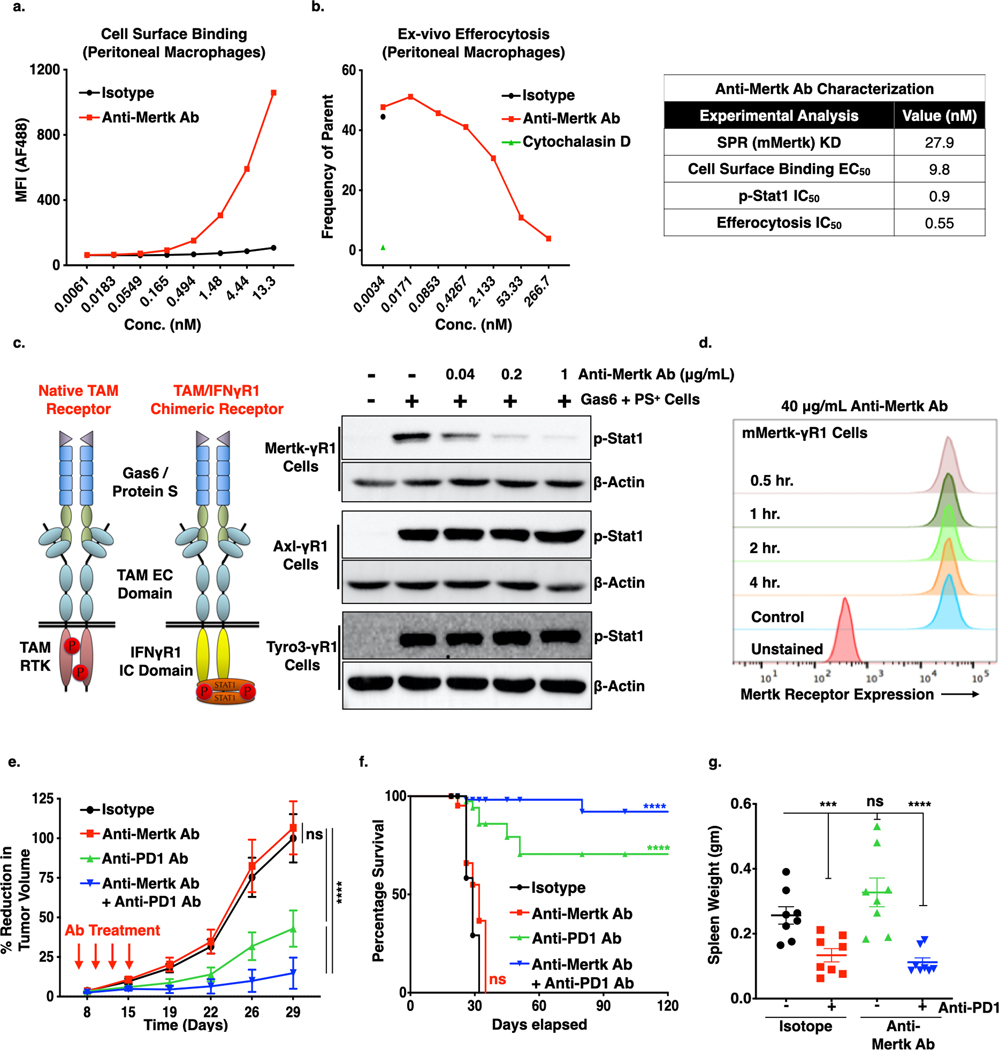
Figure 5. Effect of Anti-Mertk antibody on the Mertk receptor inhibition, efferocytosis, tumor growth and metastasis.
a. Cell surface binding analysis of Anti-Mertk monoclonal antibody (mAb) to the Mertk receptor on the peritoneal macrophages. b. In vivo efferocytosis showing decreased engulfment of apoptotic mouse thymocytes in the presence of anti-Mertk mAb as compared to isotype control. Cytochalasin D was used as a positive control (Left panel). Characterization of anti-Mertk antibody by surface plasma resonance (SPR) binding analysis, cell surface binding, p-Stat1 activation analysis in chimeric murine Mertk-γR1 cells and in vivo efferocytosis (Right tabel). d. Immunoblot analysis showing anti-Mertk antibody induced inhibition of Gas6 and apoptotic cells mediated activation Tyro3, Axl and Mertk receptors in the CHO cells expressing chimeric murine TAM-γR1 receptors. e. Flow-Cytometry based analysis showing effect of 40 μg/ml anti-Mertk ab on the Mertk receptor internalization in the CHO murine Mer-γR1 chimeric cells over the period of 4 hrs using Mertk specific flow-based antibody. f. Anti-tumor effect of anti-Mertk mAb in combination with Anti-PD1 immunotherapy. E0771 tumor bearing females C57/B16 (n=8/per group) were treated with mIgG1 Isotype control, anti-Mertk aAb (10mg/kg), anti-PD1 ab (5mg/Kg) alone and anti-Mertk ab in combination with anti-PD1 ab on day 6, 9,12, and15 and tumor growth was studied twice a week over the period of 4 weeks. g. Kaplan-Meier curve showing percentage survival of tumor bearing mice upon treatment with anti-Mertk ab and anti-PD1 immunotherapy. h. Upon sacrifice, spleens were also collected, and splenomegaly was quantified by means of spleen weight. *P<0.05, ****P<0.001. Mean values ± SD are shown (n = 8).