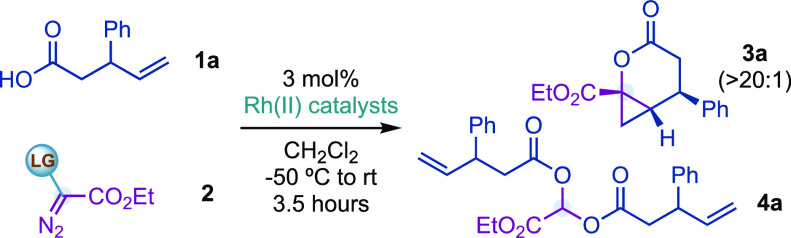
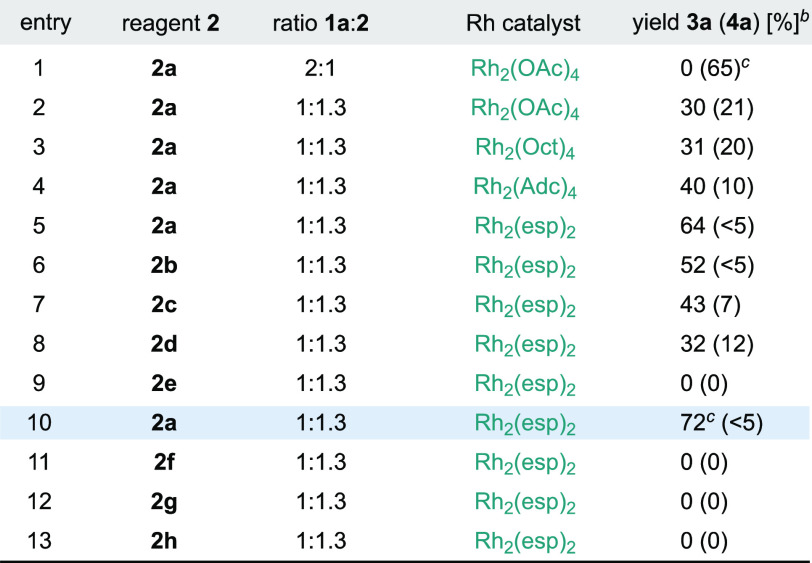
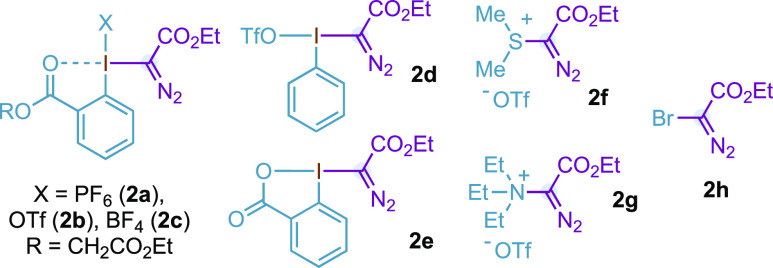
Table 1. Optimization Studiesa.
Performed at 0.1 mmol scale by addition of 1a and 2 over the Rh catalyst in CH2Cl2 at −50 °C during 30 min and then warmed to rt in 3 h.
1H NMR yields used CH2Br2 as an internal standard; a single diastereoisomer of 3a was observed (>20:1) in each entry, and 4a was obtained as an equimolecular mixture of four diastereoisomers.
NaHCO3 was added (2 equiv). oct = octanoate. adc = 1-adamantylcarboxylate. esp = α,α,α′,α′-tetramethyl-1,3-benzenedipropanoate.