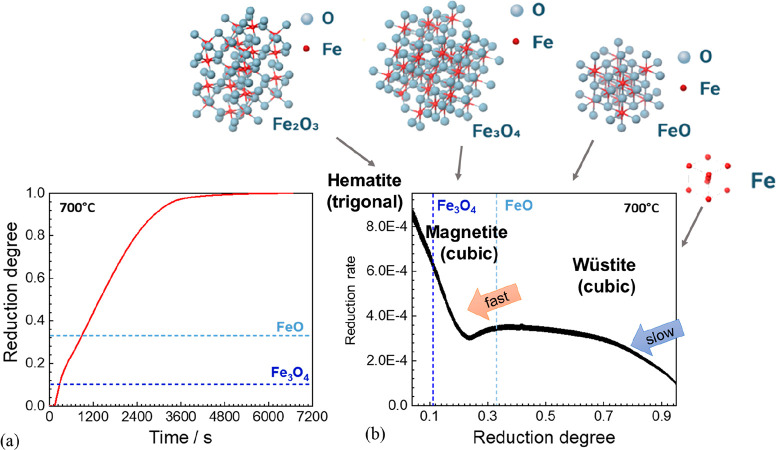
Figure 125.
Reduction kinetics of direct reduction hematite pellets at 700 °C under pure hydrogen at a flow rate of 30 liter per hour, measured by using thermogravity analysis. (a) Mass change and temperature versus time. (b) Reduction rate versus reduction degree. The end of the abrupt slope between the 0.11 and 0.15 reduction degrees marks the end of the first stage of the reduction. It is characterized by the fast transition from hematite (Fe2O3) to magnetite (Fe3O4), and the subsequent stage of decelerating reduction rates roughly ends at the local minimum of ∼0.23, indicating the Fe3O4 to FeO (wüstite) transition regime. The further reduction of the FeO into bcc Fe becomes then the rate-limiting process. The figure is reproduced in modified form with permission from ref (241) Copyright 2021, Elsevier.