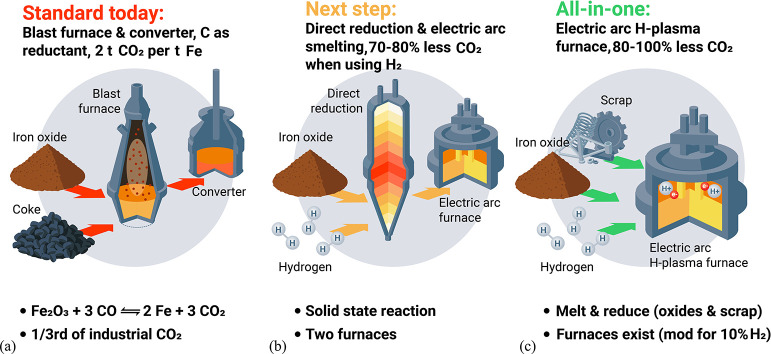
Figure 37.
(a) Steel making through the classical blast furnace and downstream basic oxygen converter route with the use of CO as reductant in the blast furnace, producing ∼1.9–2.2 tonnes CO2 per ton of steel produced. This qualifies the blast furnace process as the largest single CO2 emitter on the globe. (b) Direct reduction: solid-state reduction of hematite (or magnetite) with either methane or hydrogen (or reductant mixtures). The hydrogen-based direct reduction variant is capable of reducing the carbon footprint of iron making by 70−80% when using hydrogen from renewable production. (c) Reductant-containing plasma (e.g., hydrogen-based plasma) atmospheres in electric arc furnaces used for liquid-state oxide reduction (also called hydrogen plasma smelting). This furnace could also be additionally charged with steel scrap. This means that plasma reduction offers an all-in-one process of melting, mixing, and liquid-state reduction.