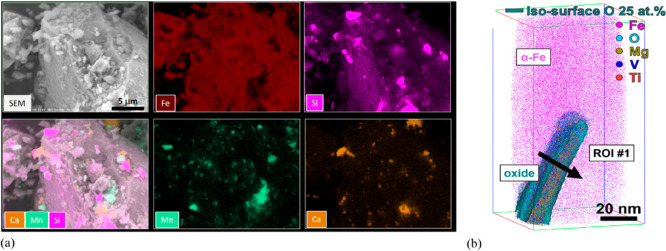
Figure 82.
Example of gangue elements in hematite ores: measurement of retained impurity content after reduction in a pure hydrogen atmosphere at 700 °C.241 (a) SEM-EDX analysis of gangue element content and distribution. (b) Atom probe tomography of nano-oxides containing gangue elements such as Mg, V, and Ti, which tend to form stable oxides. This result shows that the impurity content of iron ores can play a role for the kinetics and thermodynamics of the hydrogen-based direct reduction of such minerals and must be taken into account in the design of the reactors, in the composition of the reducing agent mixtures, in the determination of partial pressures and reaction temperatures, and in the selection of the ores. The figure is reproduced in modified form with permission from ref (241). Copyright 2021, Elsevier.