Fig. 1.
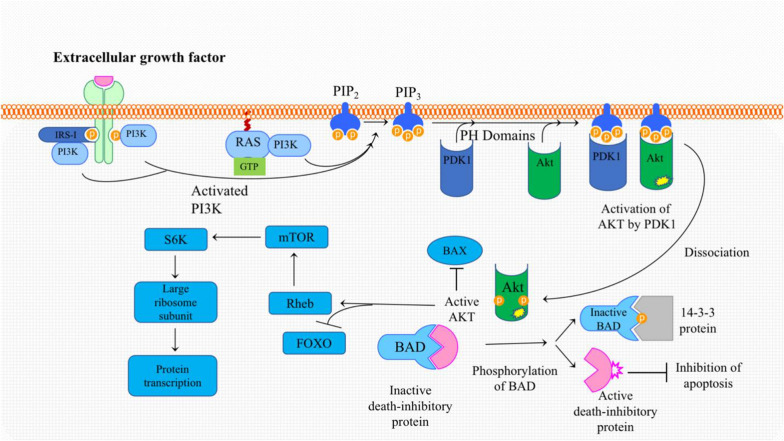
Activation of PI3K: Binding of an external ligand leads to the dimerization of receptor monomers and the heterologous autophosphorylation. Depending on the receptor, different proteins may bind to a phosphorylated domain. The insulin receptor substrate-1(IRS-1) binds to the activated IGF-1 receptor. IRS-1 serves as a binding and activation site for PI3K. In addition, PI3K may bind directly to a phosphorylated receptor tyrosine kinase, a completely different mechanism of PI3K. Activation begins with the small membrane bound GTPase Ras. By binding to active GTP-bound Ras, PI3K is activated, and migrates to the inner side of the cell membrane where it binds to phosphatidylinositol bisphosphate or PIP2. PI3K can phosphorylate PIP2 to PIP3, which can activate protein kinase B, also known as Akt. Akt binds to BAX and hinders its ability to form pores in the outer mitochondrial membrane, thus suppressing apoptosis. Moreover, Akt phosphorylates BAD leading to the release and activation of death inhibitory protein, which, as the name implies, inhibits apoptosis. Akt can also promote protein synthesis by first activating Rheb, which activates mTOR. mTOR itself interacts with and activates the translation factor S6K, thereby promoting mRNA translation and protein synthesis. In addition, Akt-induced phosphorylation of FOXO promotes the transfer of ubiquitin peptides onto the protein causing FOXO to undergoe proteasomal degradation