PNAS Howarth et al. 10.1073/pnas.0503125102. |

Fig. 8.
BirA labeling in the absence of added ATP. Unrecycled BirA is the BirA that is initially obtained from recombinant expression and contains bound biotin-AMP. Recycled BirA has had this copurifying biotin-AMP removed (see Materials and Methods). HeLa cells expressing AP-CFP-TM were incubated with 0.8 mM recycled or unrecycled BirA for 5 min, either with or without added ATP and biotin. Biotinylated proteins at the cell surface were detected with streptavidin-Alexa Fluor 568. For the 3´ sample, 2.4 mM BirA was added to the cells. A control is shown with a point mutation in AP (Ala-CFP-TM).
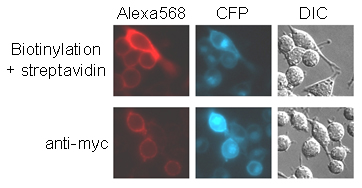
Fig. 9.
Sensitivity of BirA surface labeling. HeLa cells transfected with AP-CFP-TM were either incubated with BirA for 10 min followed by streptavidin-Alexa Fluor 568 (Upper) or incubated with anti-myc antibody followed by Alexa Fluor 568-conjugated anti-mouse antibody (Lower).

Fig. 10.
Speed of BirA surface labeling. HeLa cells transfected with AP-GluR2 were incubated with BirA for 1-60 min and stained with streptavidin-Alexa Fluor 568 for 10 min. CFP was a cotransfection marker. GluR2 with a mutation in AP was a control.

Fig. 11.
QD surface labeling. (A) Comparison of QD605- and Alexa Fluor 568-labeled cells at different exposure times. HeLa cells expressing AP-CFP-TM were biotinylated with BirA for 10 min and stained with streptavidin-Alexa Fluor 568 or streptavidin-QD605. (B) Minimizing the time for QD labeling. HeLa cells transfected with AP-CFP-TM were incubated with BirA for 1 min and then with streptavidin-QD605 for 1 min. Ala-CFP-TM is shown as a control. (C) Targeting QDs to the AP-tagged epidermal growth factor receptor (EGFR). HeLa cells expressing AP-EGFR were biotinylated with BirA for 10 min and incubated with streptavidin-QD605. The control is shown with a point mutation in AP. CFP was a cotransfection marker.

Fig. 12.
QD blinking on labeled neurons. The integrated pixel intensities of two QDs from Movie 1 ("A" and "C") were measured every 0.3 s for 12 s and plotted together with the pixel intensity of a neighboring area of equal size on the neuron. QDs "A" and "C" were immobile during this time period. Single-step disappearance and reappearance of fluorescence is indicative of single QDs, as opposed to QD clusters. Most QDs that we observed were like QD "C" and blinked infrequently, whereas some, like QD "A," blinked rapidly.
Supporting Movie 1
Movie 1.
Single-particle imaging of QDs bound to AMPA receptors. Day-10 hippocampal neurons in dissociated culture, transfected with AP-GluR2 and PSD-95-YFP, were biotinylated with BirA for 5 min, followed by incubation with streptavidin-QD605 for 2 min at room temperature. QD movement was followed at 37°C. The PSD-95-YFP image is shown first and then overlaid on the DIC image. Next, the QD605 movie (speeded up 3-fold) is shown overlaid on PSD-95-YFP. Finally, the QD605 movie is shown alone. The blinking of QDs "A" and "C" are plotted in Fig. 12, while "B" and "C" are examples of immobile QDs at PSD-95-YFP puncta.
Supporting Methods
General Materials.
Biotin, a gift from Tanabe U.S.A. (San Diego), was dissolved in DMSO at 100 mM, and working dilutions were made in PBS at 1 mM for use in reactions at a final concentration of 10 m M. ATP (Sigma) was dissolved at 100 mM in water, and the pH was gradually adjusted to 7.5. Aliquots were stored at –80°C and used once.Plasmid Construction.
Rat AMPA receptor subunits GluR1 and 2 (flop forms) in pBluescript SK(–) (Stratagene) were kind gifts from S. Heinemann (The Salk Institute for Biological Studies, La Jolla, CA). A unique NheI site was introduced 3 residues after the signal peptide cleavage site, and AP was inserted at the NheI site with the primers 5'-CTAGGGGCCTGAACGATATCTTCGAGGCCCAGAAGATCGAGTGGCACGAGG and 5'-CTAGCCTCGTGCCACTCGATCTTCTGGGCCTCGAAGATATCGTTCAGGCCC .Each gene was then subcloned into the XhoI site of CMVbipep (1) after PCR with the primers 5'-ATGCCTCGAGCCCCCGGGCTGCAGGAATTC and 5'-GTACCGGGCCCCCCCTCGAG to give AP-GluR1 or AP-GluR2. Construction of AP-CFP-TM and AP-EGFR and mutation of the lysine in AP to alanine (to give Ala-CFP-TM, Ala-EGFR, and Ala-GluR2) are described in ref. 2. pEYFP-N1-PSD95 was constructed from pEGFP-N1-PSD95, a gift from S. Okabe (Tokyo Medical and Dental University, Tokyo), by replacing GFP with YFP using the EcoRI and KpnI sites.
Cell Culture and Transfection.
HeLa cells were grown in DMEM with 10% FCS, 50 units/ml penicillin, and 50 mg/ml streptomycin and were transfected with Lipofectamine 2000 (Invitrogen), following the manufacturer’s instructions. HeLa cells were cultured overnight at 32°C and 5% CO2 before biotinylation, because this improved export to the cell surface. HeLa cells stably expressing AP-CFP-TM or Ala-CFP-TM were generated by selection with 0.5 mg/ml G418 and purification with CELLection Pan Mouse IgG Dynabeads (Dynal, Great Neck, NY) coated with anti-myc antibody (Oncogene Science). Hippocampal neurons in dissociated culture were prepared from P1-3 Sprague-Dawley rats and maintained at medium density (~150 cells·mm-2) in minimal essential medium without phenol red (Invitrogen), 5 mg/ml glucose, 200 mg/ml NaHCO3, 100 mg/ml transferrin (Calbiochem), B27 (Invitrogen), and 0.5 mM glutamine. Neurons were transfected 8-12 days later by using Lipofectamine 2000. For cotransfections, plasmids were mixed with pcDNA3-CFP-Ala (2) at a ratio of 7:1 or with pEYFP-N1-PSD95 at a ratio of 5:3.1. Duch, M., Tolstrup, A., Dalum, I., Jespersen, T., Mouritsen, S. & Pedersen, F. S. (1999) BioTechniques 26, 1032-1034, 1036.
2. Chen, I., Howarth, M., Lin, W. & Ting, A. Y. (2005) Nat. Methods 2, 99-104.