
Cary et al. 10.1073/pnas.0506289102. |
Supporting Figure 6
Supporting Figure 7
Supporting Text
Supporting Figure 8

Fig. 6. Calculation of the amount of sGC converted to the ferrous-nitrosyl species. The absorbance maximum in the electronic absorption spectrum of sGC shifts from 431 nm to 399 nm with the addition of increasing amounts of NO, indicating formation of ferrous-nitrosyl sGC. Using the extinction coefficients of sGC and ferrous-nitrosyl sGC, we generated simulated spectra of mixed populations and overlaid them on observed spectra for each addition of NO to sGC. This allowed us to calculate the percentage of ferrous-nitrosyl sGC in each mixture. The orange line is the calculated spectrum of a mixture containing 77% ferrous-nitrosyl sGC.
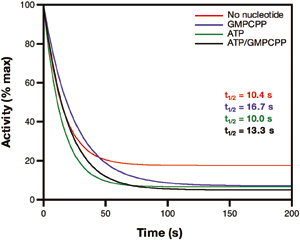
Fig.7. Deactivation of sGC-NO using a truncated globin from Mycobacterium bovis [hemoglobin (HbN)] as the NO trap. Using the HbN NO trap yields deactivation rates that are similar, regardless of the presence of nucleotide. The CO/dithionite NO trap essentially deactivates sGC within the mixing time of the experiment (data not shown). In all cases, deactivation was rapid and independent of the presence of nucleotide. Experiments were conducted as reported in Fig. 2, except for the following: For the HbN trap, the final concentration of HbN was 5 mM, and when the CO/dithionite trap was used, the reactions were carried out anaerobically in a Teflon-sealed Reacti-Vial (Pierce) and aliquots were withdrawn by using a Hamilton gas-tight syringe. Data were fit to Eqs. 1 and 2 (Materials and Methods) and are shown as a percentage of initial activity.

Fig. 8. Ferrous-nitrosyl sGC has low activity and requires excess NO for full activity. (A) Electronic absorption spectra of sGC and ferrous-nitrosyl sGC (4.7 mM). Ferrous sGC has a Soret peak at 431 nm (blue trace), and ferrous-nitrosyl sGC has a Soret peak at 399 nm (orange trace). (B) Electronic absorption spectra and activity of deactivated sGC (470 nM) with or without excess NO. sGC was activated with NO and then deactivated with an oxymyoglobin (MbO2) NO trap. The trap was separated from the sGC by anion exchange (Q resin) chromatography. The green trace is the spectrum of the deactivated sGC, and the purple trace is the spectrum of the deactivated sGC with excess NO. Data are representative of 12 repeated experiments. (C) Corresponding enzyme activities of protein samples described in B as a percentage of full activity (4,020 nmol cGMP min–1·mg–1 at 22°C). Bar colors match the colors of the spectra in A and B.
Supporting Text
Deactivation of NO-Activated Soluble Guanylate Cyclase (sGC): Correlation of Spectra and Activity.
The ~160-fold difference in rates of NO dissociation and sGC deactivation in vitro [a t1/2 of ~19 min (adjusted to 20°C) for dissociation versus t1/2 of ~7 s for deactivation] suggests that NO remains bound to deactivated sGC. To test this hypothesis, we added a NO trap to fully active sGC-NO, removed the trap, and simultaneously recorded the electronic absorption spectrum and activity of the resulting sGC species.NO in excess of the sGC heme was removed using a MbO2/Q anion-exchange trapping method as follows. sGC was treated with 3-fold excess NO for ³5 × t1/2 [assuming 1.5 eq of NO per eq of DEA/NO, and a decomposition t1/2 of ~7 min at 25°C (1)]. Formation of the ferrous-nitrosyl species was monitored at 22°C by following the shift in the Soret absorbance maximum from 431 to 399 nm. An aliquot was removed and assayed for activity before and after addition of NO. MbO2 (5-fold excess) was added, and the solution was applied to 200 ml of Tosohaas Q650M anion-exchange resin equilibrated with 50 mM Hepes (pH 7.4)/50 mM NaCl. The column was washed twice with 500 ml of 50 mM Hepes (pH 7.4)/50 mM NaCl to remove MbO2, and the bound ferrous-nitrosyl sGC was eluted with 200 ml of 50 mM Hepes (pH 7.4)/500 mM NaCl. Assays were carried out in the spectrophotometer at 22°C as follows. MbO2 trap/Q column-treated ferrous-nitrosyl sGC was placed in a cuvette, a spectrum was recorded, and reactions were initiated by addition of MgGTP, mixing, and initiation of data collection. Spectra were acquired every minute throughout the course of each assay. Final concentrations of MgCl2 and GTP were 6 mM and 3 mM, respectively, and final concentrations of sGC were 350–600 nM. Reactions were quenched after 5–6 min by the addition of 100 ml from the cuvette to 400 ml of 125 mM Zn(CH3CO2)2, to which was then added 500 ml of 125 mM Na2CO3. The cGMP formed was quantified by EIA.
The spectrum of the resulting sGC species indicates that the heme is still predominantly NO-bound (compare A and B of Fig. 8), but activity is reduced by >85% (Fig. 8C). With addition of excess NO, full activity is restored to the low-activity ferrous-nitrosyl sGC, concurrent with a slight spectral shift that indicates that the sGC heme is now 100% NO-bound. These observations, together with those of Russwurm and Koesling (2), indicate that the binding of NO to the sGC heme is not sufficient for full activation; some other factor must be required.
1. Keefer, L. K., Nims, R. W., Davies, K. M. & Wink, D. A. (1996) Methods Enzymol. 268, 281–293.
2. Russwurm, M. & Koesling, D. (2004) EMBO J. 23, 4443–4450.