Supporting information for Barnés et al. (2002) Proc. Natl. Acad. Sci. USA 99 (8), 5195–5200. (10.1073/pnas.032089399)
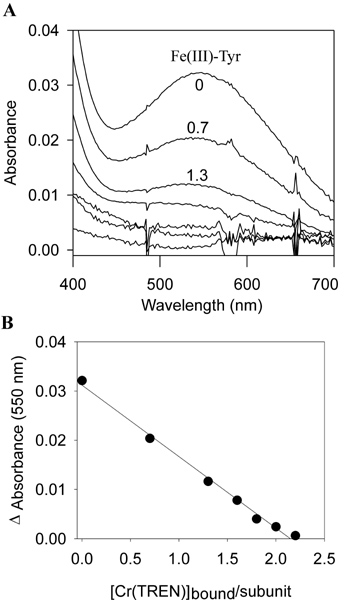
Fig. 6.
Inhibition of Fe(III) Intermediate in rH-L134P by Cr(TREN). rH-L134P (2 m M) was preincubated with Cr(TREN) (0-1.5 mM) for 24 h at 25°C to yield Cr(TREN)-rH-L134P complexes with stoichiometries of binding ranging from 0 to 2.2 Cr(TREN) per subunit. Fe(II) was then added to the Cr(TREN)-rHL134P complexes (48 Fe(II)/protein, [Fe] =0.1 mM), and the absorption of the long-lived Fe(III) ferroxidase intermediate (with Amax at 550 nm) was after Fe(II) addition. (A) The spectra of the Fe(III) ferroxidase intermediate, measured 10 seconds after Fe(II) addition, in rH-L134P-Cr(TREN) complexes containing (from top to bottom) 0, 0.7, 1.3, 1.6, 1.8, 2.0, and 2.2 Cr(TREN) per subunit, respectively (only first three stoichiometries of binding indicated in figure). (B) The inhibition of the Fe(III) intermediate (550 nm) linearly correlates to the amount of Cr(TREN) bound to rH-L134P.