Li et al. 10.1073/pnas.0508531102. |

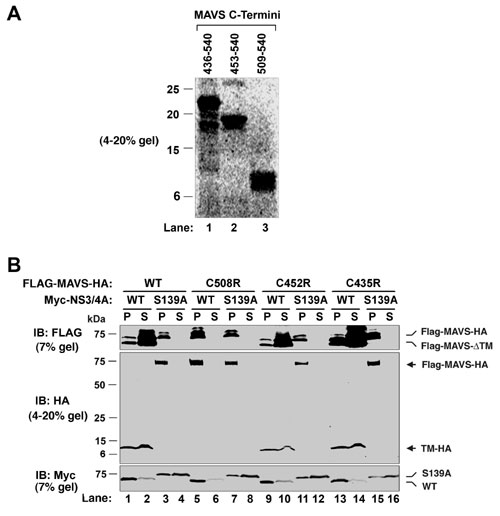
Fig. 5. Mitochondrial antiviral signaling protein (MAVS) is cleaved by NS3/4A at Cys-508, but not other C-terminal Cys residues. (A) Expression vectors encoding the C-terminal fragments of MAVS as indicated were translated in vitro in the presence of 35S-methionine. Each fragment also contains an N-terminal methionine and a C-terminal hemagglutinin (HA) tag, so that each can serve as a size marker for the C-terminal fragment of MAVS if the cleavage were to occur at Cys-435, -452, or -508 (see Fig. 2D). (B) HEK293 cells were transfected with expression constructs for NS3/4A and MAVS containing mutations at Cys residues as indicated. Cell lysates were separated into membrane pellet (P) and cytosolic supernatant (S) and then analyzed by immunoblotting.

Fig. 6. Activity and localization of NS3/4A. (A) MAVS is cleaved directly by NS3/4A in vitro. The His-6-NS3/4A protease was expressed in Escherichia coli and then purified by using nickel affinity column. The purified protease was incubated with 35S-labeled MAVS or its C508R mutant at 30°C for 2 h and then analyzed by SDS/PAGE followed by PhosphorImaging. (B) NS3/4A is localized within or proximal to the mitochondrial membrane. The expression vector encoding Flag-NS3/4A was transfected into HeLa cells and the expressed protein stained with the Flag antibody and then visualized by confocal microscopy. Cells also were stained with Mito Tracker (Upper) or an antibody against calnexin (Lower) to label the mitochondria or endoplasmic reticulum, respectively.