
Buffo et al. 10.1073/pnas.0506535102. |

Fig. 5. Amyloid plaque deposition in the neocortex of the Thy1-APPPS mice. Cresyl violet staining reveals the presence of amyloid deposits (arrows) in the neocortex of a 6-month-old Thy1-APPPS mouse (GM, gray matter; WM, white matter). (Scale bar: 50 mm.)
Fig. 6. Cortical gliotic response after a stab wound. The time course of the gliosis after a stab wound is illustrated in A. The density of fast proliferating cells that incorporated BrdUrd or were labeled with different markers in the lesioned cortex was normalized to the corresponding values of the contralateral cortex (A). The histograms in B and C describe in detail the response of astrocytes and NG2-positive precursors to the lesion: Their number significantly increases with time after stab wound, but, while NG2-expressing precursors return to control values, S100b-positive astrocytes are still significantly increased after 30 days (B, P = 0.011 at 7 days, P = 0.012 at 30 days; C, P = 0.011 at 3 days, P = 0.024 at 7 days). hs, hours; dpl, days after lesion.
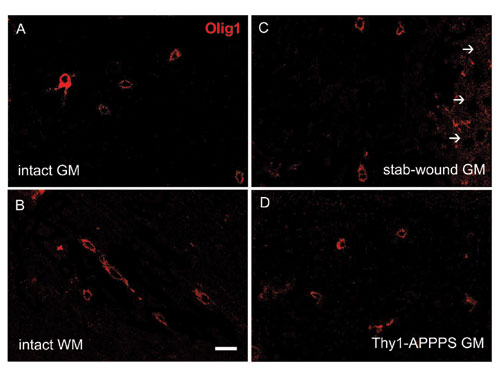
Fig. 7. Olig1 expression in the intact neocortex and in lesion conditions. The micrographs in A and B show Olig1-positive cells in the intact neocortex. The pattern of Olig1 expression at 7 days after a stab wound is illustrated in C, whereas D shows the distribution of Olig1-positive cells in a 6-month-old Thy1-APPPS mouse (GM, gray matter; WM, white matter; arrows point to the stab-lesioned track). In all cases, Olig1 staining displays a cytoplasmic localization. (Scale bar: 20 mm.)
Fig. 8. Cortical gliotic response upon amyloid plaque formation. The histograms in A illustrate the composition of fast proliferating cells (short BrdUrd pulse) in the wild-type and transgenic cortex. Also, the Thy1-APPPS cortex shows a prominent increase in the number of astrocytes and NG2-positive cells (B), whereas the increase in the oligodendrocyte number does not reach statistical significance (P = 0.005 for S100b-positive astrocytes; P = 0.007 for NG2-expressing cells and P = 0.072 for CC1-labeled oligodendrocytes). Asterisks indicate statistically significant differences. WT, wild type.
Table 1. Identification criteria for the cortical cell types
Cell type | Markers | Referred as |
Astrocytes | S100β+/NG2–/CNPase– (ref. 1) or GFAP+ | S100β+ |
Glial precursors | NG2+(S100β+) or PDGFαR+ | NG2+ |
Oligodendrocytes | CC1+/S100β–/GFAP– (ref. 2) | CC1+ |
Endothelial cells | CD31+ | Endothelial cells |
Microglia | Cd11b+ or GSA+ | Microglia |
Leukocytes | CD45+ | Leukocytes |
Neurons | NeuN+, HuC/D+, MAP2+ | Neurons |
1. Hachem, S., Aguirre, A., Vives, V., Marks, A., Gallo, V. & Legraverend, C. (2005) Glia 51, 81–97.
2. Horner, P. J., Power, A. E., Kempermann, G., Kuhn, H. G., Palmer, T. D., Winkler, J., Thal, L. J. & Gage, F. H. (2000) J. Neurosci. 20, 2218–2228.Supporting Material
Immunohistochemistry and in Situ Hybridization.
BrdUrd was stained after 2 M HCl pretreatment. Primary antibodies (see also Table 1 for cell-type identification criteria) were directed against: BrdUrd (1:10, mouse, BD Bioscience, or rat 1:200, Abcam, Cambridge, MA); glial fibrillary acidic protein (GFAP) (1:100, mouse, Dako, Glostrup, Denmark, or 1:1,000, rabbit, Sigma); S100b (1:1,000, mouse, Sigma); NG2 (1:200, rabbit, Chemicon); platelet-derived growth factor-aR (1:100, rat, BD Bioscience); 2', 3'-cyclic nucleotide 3'-phosphodiesterase (CNPase, 1:100, mouse, Sigma); Cd11-b (1:50, rat, Serotec, Oxford); lectin from Bandereia Simplicifolia (1:100, GSA, Sigma); CD45 (infiltrating leukocytes, 1:50, rat, Serotec); CC1 (1:100, mouse, Calbiochem, San Diego); CD31 (1:50, rat, Serotec); doublecortin (dcx, 1:1,000, goat, Santa Cruz Biotechnology); nestin (1:100, mouse, Hybridoma Bank, Iowa City, IA); MAP2 (1:100, mouse, Sigma), NeuN (1:75, mouse, Chemicon) or HuC/D (1:100, mouse, Molecular Probes) for neurons; Lewis-X (BD Bioscience); GFP (1:500, RDI, Flanders, NJ); Olig1 and Olig2 (respectively, 1:100, mouse and 1:40,000, rabbit, ref. 1); Mash1 (1:1,000, mouse, kindly provided by D. Anderson, Howard Hughes Medical Institute, California Institute of Technology, Pasadena, CA) and Neurogenin2 (Ngn2, 1:1,000, rabbit, kind gift of F. Guillemot, National Institute for Medical Research, London); Pax6 (1:5,000, rabbit, Chemicon); Sox10 (1:1000, guinea pig, kind gift of M. Wegner, University of Erlangen, Erlangen, Germany); Gsh2 (1:2,000, rabbit, kind gift of K. Campbell, Cincinnati Children's Hospital Medical Center, Cincinnati). Antibodies were diluted in 0.1 M PBS (0.5% Triton X-100, 10% normal serum). Appropriate species/subclass specific secondary antibodies conjugated to fluorescein isothiocyanate (FITC), tetramethylrhodamine B isothiocyanate (1:200, Jackson Laboratory), or biotin (1:200, Vector Laboratories, Burlingame, CA) were applied for primary antibody detection. Streptavidin conjugated to different fluorophores [7-amino-4-methyl coumarin-3-acetic acid (AMCA), FITC, and Texas red, Vector Laboratories] was used to visualize biotinylated antibody or GMA. Transcription factors were detected by high sensitivity tyramide signal amplification (PerkinElmer) that allowed amplification of the signal and simultaneous detection of two antibodies raised in the same animal. The specimens were observed by using a Zeiss Axioplan microscope and an Olympus FV1000 laser-scanning confocal microscopes. Sections were optically sliced in the Z plane by using 1-mm intervals, and cells were rotated in orthogonal planes to verify double labeling. Digoxigenin-labeled RNA probes for Olig2 were made, and in situ hybridization was performed on sections of perfused brains.1. Arnett, H. A., Fancy, S. P., Alberta, J. A., Zhao, C., Plant, S. R., Kaing, S., Raine, C. S., Rowitch, D. H., Franklin, R. J. & Stiles, C. D. (2004) Science 306, 2111–2115.