Pagliari et al. 10.1073/pnas.0506712102. |
Fig. 7. Heating of light membranes does not cause cytochrome c release from purified mitochondria. Heavy membranes isolated from mouse liver were further fractionated over Percoll to separate the light membranes from mitochondria. (A) Percoll-purified mitochondria were heated at 43°C for the indicated times in the absence or presence of rBcl-xL or cytosolic extract (CE, 4 mg/ml). After an additional incubation for 1 h at 37°C, samples were centrifuged, and the mitochondria (pellet) and supernatant were separated. Both were electrophoresed and probed by Western blot for the presence of cytochrome c and Hsp60, which was detected only at the pellet. (B) Light membranes (LM) were heated at 43°C for either 30 or 60 min and added to purified mitochondria for 1 h at 37°C. The samples were assayed as described in A.
Fig. 8. Heat induces cytochrome c release from Jurkat mitochondria, and Hsp60 remains soluble after heating isolated mitochondria. (A) Mitochondria were enriched from Jurkat cells and heated at 43°C for 60 min, followed by an additional incubation for 1 h at 37°C. Samples were centrifuged, and the mitochondria (pellet) and supernatant were assayed by Western blot for the presence of cytochrome c. (B) Enriched mouse-liver mitochondria were heated at 43°C for 1 h and incubated at 37°C for an additional 1 h. Where indicated, 0.75 mg/ml digitonin (Sigma) was added for 20 min on ice. The samples were centrifuged and the mitochondria (pellet) and supernatant assayed by Western blot for the presence of cytochrome c and Hsp60.
Fig. 9. Purity of recombinant Bax preparations. Recombinant full-length Bax (Left) and BaxDC (Right) were electrophoresed and visualized by Coomassie blue staining (Pierce).

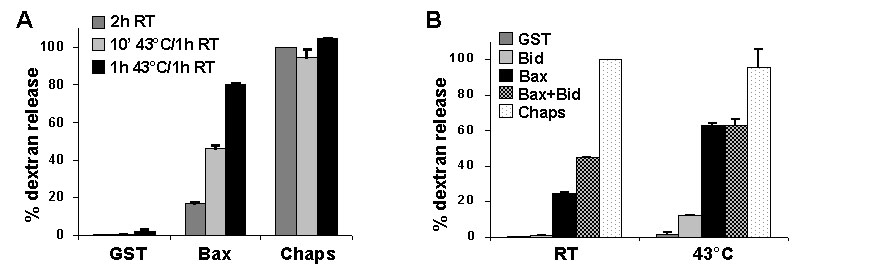
Fig. 10. Heating Bax with liposomes induces dextran release. (A) Synthetic liposomes loaded with fluorescein-dextran were incubated with 120 nM full-length Bax at 43°C for 10 min or 1 h or at room temperature for 1 h, followed by an additional 1 h incubation at room temperature. Dextran release was calculated relative to liposomes incubated for 2 h at room temperature, with GST control protein as baseline and 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (Chaps) as maximum release. (B) Liposomes were incubated with 120 nM full-length Bax and/or 45 nM N/C Bid at room temperature or 43°C for 1 h, followed by incubation for an additional hour at room temperature. Dextran release was assayed as in A. RT, room temperature.
Fig. 11. Enriched mitochondria (mito) were isolated from WT or DKO mouse embryonic fibroblasts and heated at 43°C for the indicated times. Where noted, mitochondria were incubated for an additional 1 h at 37°C. Samples were centrifuged, and the mitochondria (pellet) and supernatant were separated, electrophoresed, and probed by Western blot for the presence of Hsp60.
Supporting Methods
Cell Culture.
Mouse embryonic fibroblasts from bax–/–bak–/– double-knockout (DKO) mice and from matched WT mice were cultured at 37°C in Dulbecco’s modified Eagle’s medium, and human leukemic Jurkat T-cells (American Type Culture Collection) were maintained in RPMI Medium 1640. All media were supplemented with 10% heat-inactivated FBS, penicillin, streptomycin, and 2 mM l-glutamine.Immunoblotting.
Samples were electrophoresed by using 4–20% LongLife precast gels (Gradipore) or 4–12% Criterion XT precast gels (Bio-Rad), transferred to nitrocellulose (Hybond, Amersham Pharmacia Biosciences), and probed with antibodies to cytochrome c (7H8, 1:2,500, BD PharMingen), VDAC (31HL, 1:3,000, Calbiochem), Hsp60 (LK1, 1:2,500, Stressgen), Bak (NT, 1:1,000, Upstate Biotechnology), Bax (N-20,1:2500, Santa Cruz Biotechnology), or Bcl-x (1:2,000, BD PharMingen). After a secondary reaction with horseradish-peroxidase-conjugated anti-mouse (Amersham Pharmacia) or anti-rabbit (Pierce) antibodies, the membranes were incubated with Enhanced Chemiluminescence reagents and visualized as recommended by the manufacturer (Amersham Pharmacia).Mitochondrial Swelling.
Swelling of mitochondria was assessed by measuring a loss in light scatter at 520 nm by using a spectrophotometer. Isolated mouse-liver mitochondria suspended in MAB (100 mg/50 ml) were heated at 43°C for the indicated time and transferred to a 96-well plate. The plate reader was preheated to 37°C, and absorbance readings at OD520 were recorded every minute for 30 min. As a positive control, mitochondria were incubated with 100 mM calcium to induce swelling.Annexin V Staining.
Cells were harvested, washed in PBS, and resuspended in binding buffer (10 mM Hepes, 150 mM NaCl, 5 mM KCl, 100 mM MgCl2, and 1 mM CaCl2) containing Annexin V-FITC (1:100, Caltag). Samples were analyzed by flow cytometry and flowjo software.Preparation of Cytosolic Extracts.
Cells were harvested, washed in PBS and resuspended in one pellet volume of cell extract buffer (CEB) (50 mM Pipes, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, and 1 mM DTT) supplemented with complete proteinase inhibitors (Roche). The suspension was placed on ice for 15 min and the cells were homogenized with a Teflon dounce until 50% of cells were Trypan blue positive. Samples were centrifuged at 21,000 × g for 5 min at 4°C, and the lysate was transferred and centrifuged again for 10 min. The cytosolic extract was transferred to a fresh tube, and protein concentration estimations were performed (Bio-Rad) before storing at -80°C.Immunodepletion.
Cytosolic extract was incubated at 4°C overnight with either Bcl-x polyclonal antibody (Cell Signaling Technology) or rabbit Ig control coupled to protein A-agarose beads (Pierce). After centrifugation at 2,500 × g for 3 min to pellet the beads, the cytosolic extract was recovered, transferred, and centrifuged again to ensure complete bead removal.Production of Recombinant Proteins.
Bacteria were transformed with pET15b-BaxDC, cultured to OD600 = 0.8, induced with 0.75 mM isopropyl b-D-thiogalactoside (IPTG) for 30 min at 37°C, resuspended in buffer (20 mM Tris, 100 mM NaCl, 10% glycerol, and EDTA-free complete proteinase inhibitors) (Roche), and frozen. After thawing, bacteria were incubated with 0.2 mg/ml lysozyme for 30 min at 37°C, sonicated, and the lysate cleared by centrifugation. BaxDC was bound to Ni-CAM nickel beads (Sigma) in the presence of 10 mM imidazole overnight at 4°C. Beads were recovered by centrifugation and washed in high-salt buffer (1 M NaCl, 20 mM Tris, and 10% glycerol). Recombinant BaxD C was eluted in 250 mM imidazole and dialyzed in buffer A (20 mM Tris, 100 mM NaCl, and 10% glycerol) overnight at 4°C. Bcl-xL(G138A)DC was generated by using a QuikChange site-directed mutagenesis kit as instructed by the manufacturer (Stratagene) and isolated in the same fashion as WT Bcl-xLDC (1). Briefly, bacteria were transformed with pET29a-Bcl-xLDC or pET29a-Bcl-xLDC(G138A), grown to OD600 = 0.9, and induced with 0.5 mM IPTG for 4 h at 30°C. Bacteria were pelleted, lysed by freeze/thaw, treated with 5 mg/ml DNase and 0.2 mg/ml lysozyme for 30 min at 37°C, and sonicated. The lysate was cleared by centrifugation. Bcl-xL protein was affinity purified by binding to a 2-ml nickel-chelating column and eluted by using an imidazole gradient (0–250 mM). Fractions containing the appropriate size recombinant proteins were pooled, dialyzed overnight at 4°C against buffer A, aliquoted, and stored at –80°C. Purity of the protein preparations was assessed by electrophoresis and Coomassie (Pierce) staining (for Bax preparations, see Fig. 9).1. Bossy-Wetzel, E. & Green, D. R. (1999) J. Biol. Chem. 274, 17484–17490.