Hansen et al. 10.1073/pnas.0509549102. |

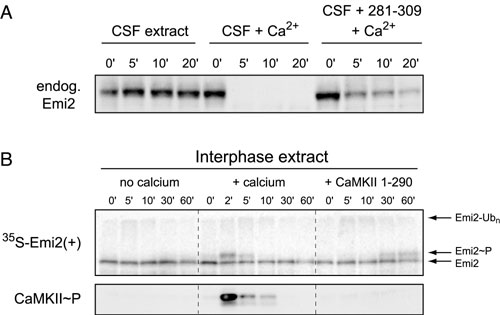
Fig. 5. Ca2+/calmodulin-dependent protein kinase II (CaMKII) is required for Emi2 destruction in cytostatic factor (CSF) extract but is insufficient to drive Emi2 destruction in interphase extract. (A) CSF extracts, untreated or preincubated with CaMKII 281-309 inhibitory peptide, were stimulated with Ca2+ addition. Stability of endogenous Emi2 was monitored by immunoblotting. (B) Radiolabeled in vitro-translated (IVT) Emi2 was incubated in interphase extract supplemented with Ca2+ or IVT active CaMKII and monitored by autoradiography.

Fig. 6. Saturation of the Emi2 destruction pathway prevents Ca2+-induced cyclin degradation. A maltose-binding protein (MBP) fusion of the Emi2 N terminus was added to CSF extract at a final concentration of 1 mM, 300 nM, or 100 nM. Ca2+-induced destruction of Emi2 and cyclin B was monitored by immunoblotting.

Fig. 7. The 192RSST and 333RLST motifs of Emi2 can each be phosphorylated in vitro by CaMKII. MBP fusions of Emi2N variants were phosphorylated in vitro by CaMKII as described in Materials and Methods, except with 0.2 mM ATP and in the presence of 0.25 mCi/ml [g-32P]ATP (1 Ci = 37 GBq). Reaction products were analyzed by electrophoresis, autoradiography, and densitometry.

Fig. 8. Ca2+-induced destruction of human Emi2 requires its 231KTST motif but not its 383RLST motif. Indicated variants of radiolabeled IVT human Emi2 protein were incubated in CSF extract with calcium addition. The 231-4* mutation is 231KTST® ATSA, and the 383-6* mutation is ST385AA. Emi2 stability was monitored by autoradiography.
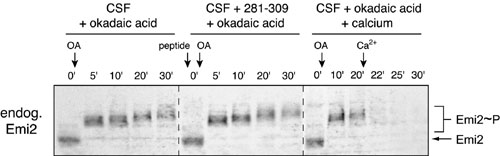
Fig. 9. Endogenous Emi2 and IVT Emi2 respond similarly to okadaic acid treatments. CSF extracts were treated with 281-309 peptide, okadaic acid, and Ca2+ as indicated, and as in Fig. 4 B and C. The stability and gel mobility of endogenous Emi2 were monitored by immunoblotting.