Kadirvelraj et al. 10.1073/pnas.0602815103. |
Supporting Table 3
Supporting Table 4
Supporting Movie 1
Supporting Table 5
Supporting Table 6
Supporting Figure 5
Supporting Figure 6
Supporting Figure 7

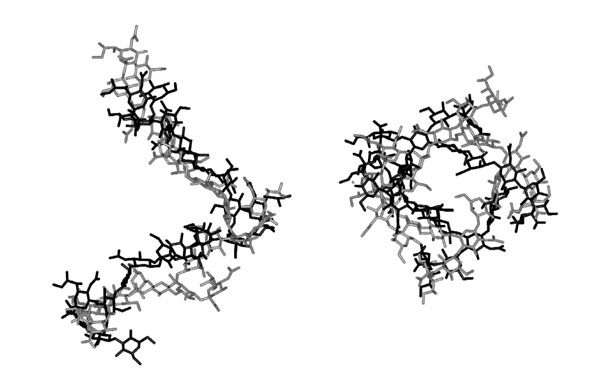
Fig. 5.
A superimposition of the average CPS conformations present in the immune complex (black) and free in solution (gray).
Fig. 6.
Electrostatic potential surface of the binding site of Fv. The molecular surfaces are colored according to electrostatic potential. Positive potential is shown in blue, and negative potential is in red; the contouring value of the potential is in kT/e. The image was drawn using PYMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA).
Fig. 7.
Superimposition of the ribbon structures of the backbones of VH1A7R (blue) and VH1NSN (red) with the H3 loops highlighted in orange.Movie 1.
MD simulation of model immune complex between Fv1B1 and CPS from GBSIII (L ® H threading).H-bond* | |||
CPS atom | Fv atom | Occupancy, % | Distance, Å |
Glc-I-O2 | Q6-O | 40.3 | 3.2 |
Gal’-II-O6 | E159-Oε1 | 31.8 | 3.1 |
Glc-II-O3 | N173-Oδ1 | 71.4 | 3.3 |
Glc-III-O6 | V99-Oa | 38.7 | 3.1 |
Glc-IV-O6 | L96-O | 33.0 | 3.2 |
Gal-V-O2 | Y37-OH | 67.9 | 3.4 |
Gal’-V-O2 | D213-Oδ1 | 53.1 | 3.1 |
Neu5Ac-V-O4 | G139-O | 61.2 | 3.2 |
Neu5Ac-V-O4 | Y145-OH | 33.5 | 3.6 |
GlcNAc-II-C=O | G157-N | 86.2 | 3.1 |
Glc-II-O3 | N173-Nδ2 | 76.3 | 3.4 |
Gal-III-O4 | A174-N | 99.7 | 3.3 |
Neu5Ac-V-O1a | R210-NH1 | 90.1 | 2.8 |
Neu5Ac-V-O1b | R210-NH2 | 88.6 | 3.0 |
Gal’-V-O3 | R210-NH2 | 65.1 | 3.6 |
Neu5Ac-V-O1b | Y214-OH | 98.6 | 3.0 |
Glc-I-O3 | Q6-N | 31.4 | 3.6 |
Gal’-I-O6 | L9-N | 66.6 | 3.3 |
Neu5Ac-IV-O4 | Y37-OH | 53.7 | 3.2 |
GlcNAc-V-C=O | R55-NH1 | 53.0 | 2.9 |
Gal-IV-O6 | V99-N | 75.4 | 3.3 |
Ring stacking (LH) | |||
CPS residue | Fv residue | θ, ° | Distance |
Glc-IV | Y37 | 156 | 5.9 |
Glc-V | Y145 | 152.5 | 5.4 |
Gal-V | Y212 | 148.3 | 6.3 |
GlcNAc-V | Y212 | 138.4 | 4.9 |
*Contacts involving residues in CDR loops are in boldface type.
H-bond* | |||||
CPS Atom | Fv atom | Occupancy, % | Distance, Å | ||
GlcNAc-I-O2 | G139-Oa | 75.2 | 3.0 | ||
GlcNAc-III-O6 | H98-ND1 | 30.6 | 3.5 | ||
GlcNAc-IV-O6 | N173-OD1 | 37.3 | 3.2 | ||
Gal-V-O2 | D1-OD1 | 41.6 | 3.2 | ||
GlcNAc-V-N2 | F103-O | 57.0 | 3.4 | ||
Gal’-I-O6 | Q114-N | 33.6 | 3.4 | ||
Gal-I-O4 | G139-N | 30.0 | 3.4 | ||
GlcNAc-I-O2N | G139-N | 44.6 | 3.1 | ||
Glc-IV-O6 | A174-N | 53.6 | 3.6 | ||
Gal-II-O6 | R210-NH1 | 34.5 | 3.2 | ||
Glc-I-O3 | R210-NH1 | 62.9 | 3.5 | ||
Glc-I-O3 | R210-NH2 | 31.2 | 3.2 | ||
Gal-IV-O6 | Q27-NE2 | 32.9 | 3.2 | ||
Glc-II-O2 | R55-NH1 | 42.1 | 3.5 | ||
Glc-II-O3 | R55-NH2 | 43.8 | 3.4 | ||
Glc-III-O6 | V99-N | 99.5 | 3.2 | ||
Ring Stacking (HL) | |||||
CPS residue | Fv residue | θ, ° | Distance | ||
Glc-I | Y145 | 135.2 | 6.7 | ||
GlcNAc-II | Y212 | 136.8 | 6.6 | ||
*Contacts involving residues in CDR loops are in boldface type.
Table 5. Predicted H-bond occupancies and distances observed in the MD simulation of the Fv1B1:GBSIII CPS complex
CPS atom | Fv atom | Occupancy, % | Distance, Å |
Gal-I-O4 | T5-Og1 | 86 | 3.3 |
Gal-II-O6 | M4-O | 33 | 3.4 |
Gal-II-O6 | F103-O | 34 | 3.6 |
Glc-II-O6 | D1-Od1 | 47 | 3.2 |
Glc-II-O3 | N173-Od1 | 29 | 3.4 |
Gal-III-O4 | Y172-O | 22 | 3.4 |
Glc-IV-O6 | L96-O | 36 | 3.5 |
Neu5Ac-IV-O4 | Y37-OH | 31 | 3.6 |
Gal'-V-O2 | D213-Od1 | 56 | 3.7 |
Gal'-V-O2 | D213-Od2 | 93 | 2.9 |
Neu5Ac-V-O4 | Y145-OH | 40 | 3.3 |
Gal'-V-O2 | Y145-OH | 27 | 3.3 |
Glc-II-O3 | N173-Nd2 | 46 | 3.6 |
Gal-III-O4 | A174-N | 96 | 3.3 |
GlcNAc-I-O2N | T7-Og1 | 21 | 3.6 |
Gal-I-O | R24-Ne | 22 | 3.5 |
Gal-I-O | R24-NH2 | 21 | 3.5 |
Neu5Ac-IV-O4 | N35-Nd2 | 34 | 3.5 |
Neu5Ac-IV-O1A | N35-Nd2 | 83 | 2.9 |
Neu5Ac-IV-O1B | N35-Nd2 | 40 | 3.5 |
Neu5Ac-IV-O1A | R55-NH2 | 21 | 3.1 |
Neu5Ac-IV-O4 | R55-NH2 | 58 | 3.2 |
Gal-III-O6 | Y101-N | 95 | 3.3 |
Contacts involving residues in CDR loops are in boldface type.
Table 6. Predicted H-bond occupancies and distances observed in the MD simulation
of the Fv1B1:Pn14 CPS complex
CPS atom | Fv atom | Occupancy, % | Distance, Å |
Glc-I-O2 | Q6-O | 50 | 3.2 |
Gal-II-O6 | F103-O | 36 | 3.1 |
Glc-II-O3 | N173-Od1 | 52 | 3.3 |
Glc-III-O6 | V99-O | 33 | 3.1 |
Gal-IV-O6 | V99-O | 24 | 3.4 |
Glc-IV-O6 | L96-O | 25 | 3.5 |
Gal-V-O2 | Y37-OH | 44 | 3.5 |
GlcNAc-V-N2 | Y54-OH | 58 | 3.4 |
Gal'-V-O2 | D213-Od1 | 36 | 3.1 |
Gal'-V-O2 | Y145-OH | 32 | 3.3 |
GlcNAc-II-O2N | G157-N | 36 | 3.1 |
Glc-II-O3 | N173-Nd2 | 74 | 3.5 |
Gal-III-O4 | A174-N | 97 | 3.3 |
Gal'-V-O2 | R210-NH2 | 26 | 3.4 |
Glc-IV-O6 | W212-Ne1 | 35 | 3.5 |
Gal'-I-O6 | L9-N | 52 | 3.3 |
Gal-V-O2 | Y54-OH | 23 | 3.3 |
GlcNAc-V-O2N | Y54-OH | 26 | 3.1 |
GlcNAc-V-O2N | R55-NH1 | 62 | 2.9 |
Contacts involving residues in CDR loops are in boldface type.