
Hicks et al. 10.1073/pnas.0602382103. |
Supporting Figure 7
Supporting Figure 8
Supporting Table 1
Supporting Figure 9
Supporting Figure 10
Supporting Figure 11
Supporting Figure 12

Fig. 7. SR/CR mice repeatedly challenged with S180 retained tumor resistance. When 20 SR/CR mice were challenged daily with S180 injections and the infiltrating cells were continuously depleted, 19 of 20 developed solid tumors. When the depletion ceased, 17 of 19 mice underwent tumor regression within 2 weeks.
Fig. 8. Resistance to S180 cells does not require PMN, NK, or MF. SR/CR mice were depleted of NK by three i.p. injections of 500 mg of anti-NK1.1 antibody. (A) Depletion was verified by flow cytometry, comparing NK cell percentages before and after injection of antibody. PMN were similarly depleted from SR/CR mice by injection of anti-Ly6G antibody and verified by flow cytometry. (B) Alternatively, SR/CR mice were depleted of MF by injection of 25 mg/kg 2-chloroadenosine followed by challenge with 2 ´ 107 S180 cells. (C) The mice that were depleted of PMN, NK, or MF did not show an increase in tumor development over control SR/CR mice. Tumorigenicity of the S180 cells was demonstrated by injection in WT control mice.

Fig. 9. Populations of immune cells were purified and analyzed for their independent abilities to destroy S180 target cells in vitro. (A) Total immune cell infiltration in response to S180 challenge is composed of multiple populations of leukocytes. (B) PMN were isolated from the peritoneal cavity 1 day after stimulation with 3% thioglycollate. Cells were purified from contaminating MF by allowing MF to adhere in cell culture and verified by hematoxylin stain. (C and D) NK were isolated from S180-stimulated peritoneal infiltrate by incubating with anti-DX5 magnetic beads. NK were then isolated over a magnetic column. Cells are shown here by phase contrast and NK1.1 fluorescence. (E and F) MF were isolated from the peritoneal cavity 3 days after stimulation with 3% thioglycollate by adherence to plastic in cell culture. Purification was verified by phase-contrast microscopy and F4/80 expression. (G and H) CD8+ T cells were purified from total splenocytes by labeling with rat anti-mouse CD8 antibody and goat anti-rat IgG. Positively labeled cells were purified over a magnetic column and verified for purity by phase-contrast microscopy and CD8 expression.
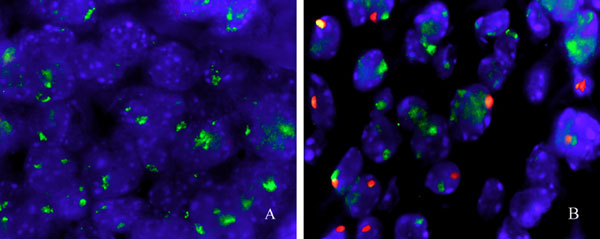
Fig. 10. Presence of male donor leukocytes in regressing S180 tumors. Shown is fluorescent in situ hybridization staining of X (green) and Y (red) chromosomes in established S180 tumors that received no AT (A) or that received SR/CR leukocyte AT 24 days before fixation (B). (Original magnification: ´500.)

Fig. 11. Presence of donor cells in AT recipients. (A) Rag1–/– female recipients were injected i.p. with male SR/CR donor immune cells for a total of eight injections. The female recipient and control Rag1–/– mice who received no male leukocytes were then challenged with 106 S180 cells i.p. The peritoneal cells were retrieved by peritoneal washing and probed for the presence of X (green) and Y (red) chromosomes. Cells from the control female mouse showed no Y chromosomes and a small number of total cells. (B) All recipients had significantly more cells in the peritoneal cavity and also displayed cells with a Y chromosome. (Original magnification: ´500.)

Fig. 12. Mixed donor cells into immune-competent recipient. Mixed cancer-infiltrating leukocytes from SR/CR mice were transferred into 10 immune-competent F1 recipients. Mice were challenged for cancer resistance by i.p. injection of S180 cells immediately after AT. Recipients were again challenged 2–6 months after AT and 6–18 months after AT for long-term cancer resistance.
Animal identification | In vitro S180 killing | In vivo S180 resistance |
1 | – | – |
2 | + | + |
3 | – | – |
4 | – | – |
5 | + | + |
6 | + | + |
7 | – | – |
To identify SR/CR pups before challenge with cancer cells, littermates were injected with 3% TG. After 3 days, the infiltrating MΦ were recovered and analyzed for their abilities to target and destroy S180 cancer cells in vitro. Pups with effective MΦ killing corresponded to pups that subsequently resisted tumor challenge in vivo with 106 S180 cells.