Stankoff et al. 10.1073/pnas.0600769103. |
Supporting Figure 8
Supporting Text

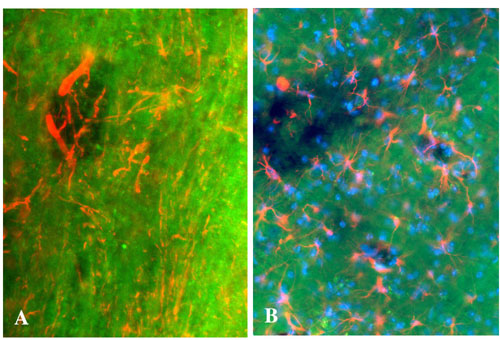
Fig. 8. Visualization of neurons, astrocytes, and Hoechst-positive nuclei in EAE demyelinated lesions. BMB (40 mg/kg) was injected i.p. to mice at day 21 after induction of EAE, and animals were perfused 4 h later. (A) Combination of BMB staining (green) with an immunolabeling of axons (2F11 antibody, red). (B) Combination of BMB staining (green) with an immunolabeling of astrocytes (anti-GFAP antibody, red) and a Hoechst staining (blue). Note the presence of denuded axons, astrocytes, and nuclei in the lesions. (Magnification: ´200.)
Supporting Text
BMB Synthesis. Reagents were purchased from Aldrich and used without further purification. The proton NMR spectra were obtained at 400 MHz on either a Bruker DPX-300 (QNP probe), DPX-400 (QNP probe), or AVANCE-400 (ATM broad-band probe) high-resolution NMR spectrometers using 5-mm NMR tubes (Wilmad 528-PP) in CDCl3 (Aldrich or Cambridge Isotope Laboratories, Cambridge, MA) solution at 25°C.
Synthesis of 1, 4-Bis(Bromomethyl)-2-Methoxy-Benzene (i)
. We added N-bromosuccinimide (34.5 g, 190 mmol) and 5 mg of benzoyl peroxide to a solution of 2,5-dimethylamisole (12.5 g, 92 mmol) in anhydrous carbon tetrachloride (600 ml). The reaction solution was refluxed under argon and visible light irradiation for 30 min. After cooling to room temperature, the precipitates were filtered and the resulting solution was concentrated under reduced pressure. The residue was recrystallized from hexanes to afford the product 1,4-bis(bromomethyl)-2-methoxy-benzene (12 g, 44%) as a white needle crystal. 1H-NMR (CDCl3, 300 MHz) d (ppm): 3.90 (s, 3H, OCH3); 4.49 (s, 2H, CH2Br); 4.56 (s, 2H, CH2Br); 6.93 (s, H, 6-H, ArH); 6.97 (dd, J1 = 1.57 Hz, J2 = 7.69 Hz, 1H, 4-H, ArH); 7.32 (d, J = 1.67 Hz, 1H, 3-H, ArH).Synthesis of 1,4-Bis(Diethoxy-Phosphonylmethyl)-2-Methoxy-Benzene (ii)
. A reaction mixture consisting of 1,4-bis(bromomethyl)-2-methoxy-benzene (i, 11 g, 37.4 mmol) and triethyl phosphite (18.6g, 112 mmol). was refluxed for 6 h. After cooling to 0°C, the crude product precipitated out. Filtration under reduced pressure afforded the product 1,4-bis(diethoxy-phosphonylmethyl)-2-methoxy-benzene (ii, 8.5g, 56%) as a white solid. 1H NMR (CDCl3, 300 MHz) d (ppm): 1.22~1.28 (m, 12H, CH3CH2O); 3.13 (d, J = 20.82 Hz, 2H, CH2P); 3.22 (d, J = 21.60 Hz, 2H, CH2P); 3.85 (s, 3H, OCH3); 3.98~4.09 (m, 8H, OCH2); 6.84 (d, J = 7.83 Hz, 1H, 3-H, ArH); 6.87 (s, 1H, 6-H, ArH); 7.26 (dd, J1 = 2.45 Hz, J2 = 8.31 Hz, 1H, 4-H, ArH).Synthesis of 1,4-Bis (4-Nitrostyryl)-2-Methoxy-Benzene (iii)
. To a solution of 1,4-bis(diethoxy-phosphonylmethyl)-2-methoxy-benzene (ii, 2,1 g, 2.4 mmol) in anhydrous tetrahydrofuran (THF) (40 ml) we added NaH (0.13 g, 5.6 mmol). The reaction mixture was stirred at 70°C for 1 h under argon light. The solution of 4-nitrobenzaldehyde (0.74 g, 4.9 mmol) in anhydrous THF (20 ml) was added dropwise to the reaction mixture. The mixture was refluxed for another 2 h. After cooling to room temperature, the reaction was quenched with water and then concentrated under reduced pressure. The residue was recrystallized from CH2Cl2 to afford the product 1,4-bis(4-nitrostyryl)-2-methoxy-benzene (iii, 0.53g, 54%) as a red needle crystalline structure. 1HNMR (CDCl3, 300 MHz) d (ppm): 4.02 (s, 3H, OCH3); 7.06~7.28 (m, 6H, ArH); 7.65~7.70 (m, 5H, ArH), 8.19~8.28 (m, 4H, ArH).Synthesis of 1,4-Bis(4-Aminostyryl)-2-Methoxy-Benzene (iv)
. Tin chloride portioning (80 mg) was added to the suspension of 1,4-bis(4-nitrostyryl)-2-methoxy-benzene (iii, 17 mg, 0.04mmol) in ethanol (5 ml), and the reaction mixture was stirred overnight at 80°C. Solvents were then removed under reduced pressure. The residue was dissolved in ethyl acetate, washed with 2.5% NaOH, and saturated in a NaCl solution. After drying with Na2SO4 to remove the solvents, the crude product was purified by flash chromatography (hexanes/ethyl acetate 1:1) to give 1,4-bis(4-nitrostyryl)-2-methoxy-benzene (iv, 11.5 mg, 80%) as a pale yellow solid. 1HNMR (CDCl3, 300 MHz) d (ppm): 3.72~3.76 (br, 4H, 2NH2); 3.95 (s, 3H, OCH3); 6.68 (d, J = 2.85 Hz, 2H, CH (2-NH2)); 6.71 (d, J = 2.86 Hz, 2H (2-NH2)); 6.95 (s, 1H, CH (ortho-OCH3); 7.01~7.03 (m, 2H, ArH); 7.08~7.11 (m, 2H, ArH); 7.35~7.40 (m, 5H, ArH); 7.55(d, J = 7.91 Hz, 1H, ArH). HRMS (EI): 343.4 (M + 1).For the in vivo studies, compound iii was labeled with positron-emitting 11C, and the methoxy group was first removed to give (E,E)-1,4-bis(4'-nitro-styryl)-2-methoxy-benzene. Subsequent reduction of the nitro groups yielded (E,E)-1,4-bis(4'-amino-styryl)-2-hydroxy-benzene product.
Radiosynthesis of [11C]BMB
. BMB has been radiolabeled with carbon-11 (T1/2 : 20.38 min) in the 2-position through radiomethylation by using the corresponding hydroxyl precursor [(E,E)-1,4-bis(4[prime]-amino-styryl)-2-hydroxy-benzene (HO-BMB)] and [11C]methyl triflate as the highly efficient methylation reagent (1). The experimental procedures included the following steps: (i) [11C]Methyl triflate was trapped at room temperature into 300 ml of DMSO solution containing 0.5-1.0 mg of HO-BMB and 5 ml of a 3M solution of NaOH in water (~5 eq.). (ii) The reaction mixture was heated at 110°C for 2 min, followed by evaporation of the solvent to dryness (at 110°C, using a helium stream for 1-2 min). (iii) The residue was dissolved in 0.5 ml of the HPLC mobile phase and loaded onto a semipreparative HPLC (SymmetryPrep C-18, Waters, 300 × 7.8 mm) for purification. Typically, starting from a 1.5 Ci (55.5 GBq) [11C]carbon dioxide production batch, 15-30 mCi (0.55-1.11 GBq) of [11C]BMB were obtained with a total synthesis time of 35 min after the end of bombardment. For in vivo imaging studies in baboon, [11C]BMB was formulated based on the following procedure: The HPLC-purified fraction containing the radiotracer was diluted with water and the resulting solution was passed through a C18 Sep-pak cartridge. The cartridge was then washed with water, partially dried with nitrogen, and finally eluted with ethanol. The solution was then sterile-filtered and diluted with physiological saline, resulting in a clear and colorless solution with a pH between 5 and 7. As demonstrated by HPLC analysis, the so-obtained radiotracer appeared to be >95% chemically and radiochemically pure.Binding of BMB to Myelin Fractions
. Rats (30-45 days) were asphyxiated with CO2. The brains (three per experiment) were removed and placed into 12 ml of ice-cold 0.32 M sucrose in a 15-ml Dounce homogenizer. All sucrose solutions were buffered with 10 mM Hepes buffer, pH 7.4, and 3 mM DTT. Brains were homogenized with five to eight strokes with a loose pestle followed by five to eight strokes with a tight pestle. The solution was then transferred to tubes and centrifuged at 20 ´ g (4°C) for 10 min. The resulting supernatant was carefully removed and transferred into Beckmann tubes that were previously filled with 3.6 ml of 2.80 M sucrose and mixed thoroughly. After mixing, the 0.85 M sucrose homogenate from one rat brain was transferred in equal parts to two ultracentrifuge tubes (each containing 2 ml of 1.4 M sucrose) and each sample was carefully overlaid with 0.25 M sucrose filling the tube. The tubes were spun in an SW 40 rotor in a Beckman ultracentrifuge for 2.5 h at 4,200 ´ g (4°C). The tubes were removed and the 0.25-M sucrose layer was removed and discarded. The myelin fraction was collected at 0.25 M/0.85 M sucrose interface as was the material that accumulated at the 0.85 M 0/1.4 M sucrose interface. Both myelin and pellet fractions were washed with Hepes buffer (7-8 ml) three times before the samples were resuspended in 5 ml of buffer, frozen, and kept in a –80°C freezer for future use. The protein concentrations of myelin and pellet were determined by Bio-Rad Protein Assay.In the spectrophotometric binding assay, 800 ml of a BMB solution (12.5 mM) dissolved in 10% DMSO buffer solution containing 10 mM MgCl2 and 10 mM PBS (pH 7.4) was incubated with isolated myelin or pellets membranes at different protein concentrations. Each tube contained 10 mM BMB, 10% DMSO buffer, and membrane fraction in a final volume of 1 ml. After incubation at room temperature for 1 h, the free and bound BMB was separated by centrifugation at 3,824 ´ g for 10 min. The supernatant was collected and the UV absorption of free BMB was determined by UV spectrometer at 380 nm. The concentration of free BMB was obtained by comparison to a standard curve. In parallel, binding to nonmyelin membrane was determined by using material that accumulated at the 0.85 M/1.4 M sucrose interface. All assays were performed in triplicate.
1. Nagren, K., Muller, L., Halldin, C., Swahn, C. G. & Lehikoinen, P. (1995) Nucl. Med. Biol. 22, 235-239.