Robenek et al. 10.1073/pnas.0600795103. |
Supporting Figure 4
Supporting Figure 5
Supporting Text

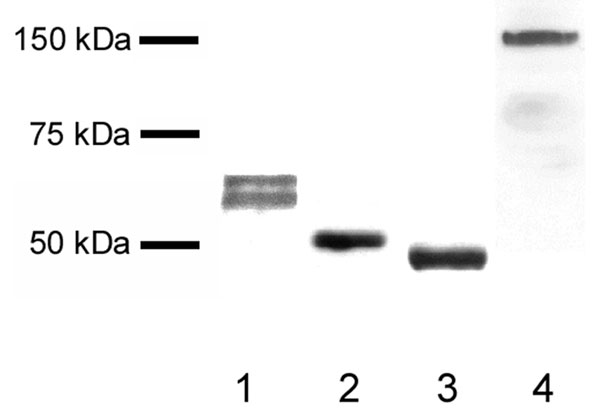
Fig. 4. Western blots demonstrating that each of the antibodies binds specifically to its target protein. Acetone-precipitated milk proteins were separated electrophoretically, blotted, and probed with anti-butyrophilin (lane 1), anti-adipophilin (lane 2), anti-TIP47 antibody (lane 3), and anti-xanthine oxidoreductase antibody (lane 4).
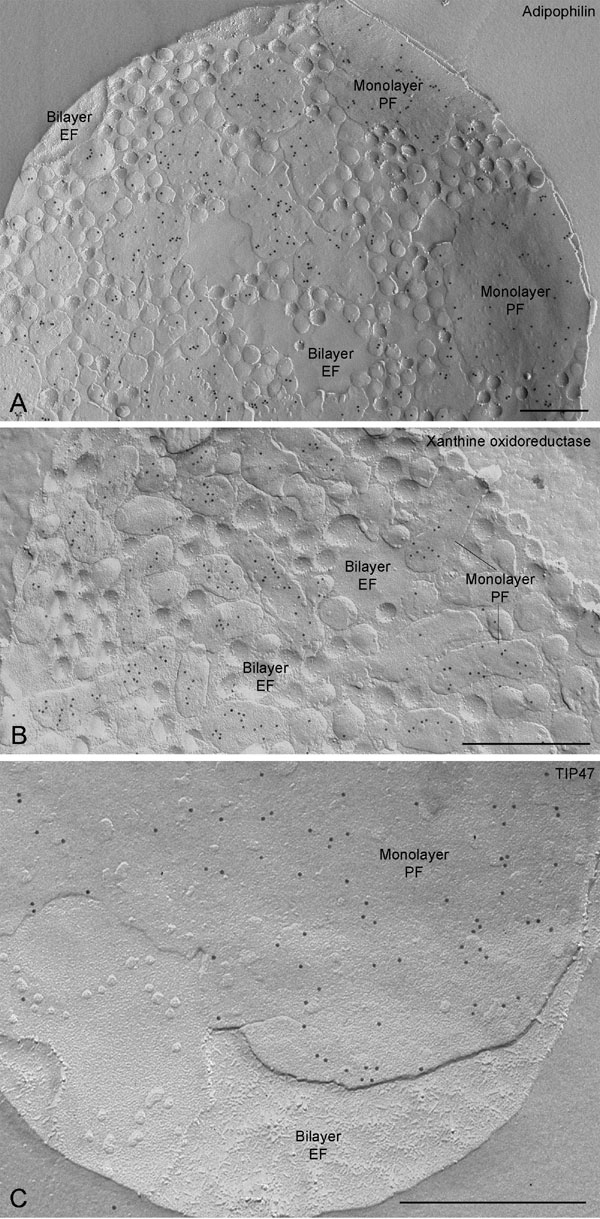
Fig. 5. Concave fractures of milk fat globules reveal adipophilin (A), xanthine oxidoreductase (B), and TIP47 (C) labeling on the monolayer P-face (PF). No label is present on the bilayer E-face (EF). (Scale bars, 0.5 mm.)
Supporting Text
Antibodies.
The first antibody to butyrophilin was directed to both of two synthetic peptides of human butyrophilin, RTSKGEKFPSTSESRC in the exoplasmic domain (N-terminal) and ERPRERRNEFSSKERC in the cytoplasmic tail region (C-terminal) and raised in rabbit (Eurogentec). The specificity of the antibody was confirmed by probing Western blots of electrophoretically separated proteins of supernatants of acetone-extracted human milk. The second antibody, raised in Guinea pig, was to whole bovine milk fat globule envelope butyrophilin isolated and purified using SDS/PAGE. The specificity of the antiserum was verified in Western blots of electrophoretically separated milk fat globule envelope proteins using standard procedures, and the efficacy of the antibody for freeze-fracture cytochemistry was confirmed. Labeling patterns with this antibody were the same as those with the antibody to human butyrophilin (data not shown). Secondary antibody-gold conjugates used for freeze-fracture immunocytochemistry were 6, 12, or 18 nm in size (Jackson ImmunoResearch).Immunofluorescence Microscopy.
One part of fresh human milk was fixed with seven parts of 2% paraformaldehyde on slides and the mixture was dried. Milk fat globule envelopes were permeabilized with 0.5% Triton X-100 in PBS and blocked with 1% BSA in PBS overnight before immunolabeling with adipophilin, TIP47, butyrophilin, or xanthine oxidoreductase antibodies. Labeled adipophilin was visualized with Cy2-conjugated goat anti-mouse IgG, TIP47 with a Cy3-conjugated donkey anti-Guinea pig antibody, butyrophilin with Cy2-conjugated or Cy3-conjugated goat anti-rabbit IgG and xanthine oxidoreductase with a Cy3-conjugated donkey anti-Guinea pig antibody. The preparations were mounted in fluorescent mounting medium (Dako Cytomation) and photographed in a laser confocal microscope (Zeiss).Freeze-Fracture Immunocytochemistry.
Immunolabeling with these antibodies was specific with virtually no background. Controls were carried out using an antibody to caveolin-1 and several antibodies to proteins previously described as not present in lipid droplets including Lamp-1, connexin43, LAP2b and emerin (data not shown). Specific labeling of milk secretory granules and milk fat globules and their envelopes was never found with these irrelevant antibodies. The preparations were studied in the electron microscope. The observations on milk fat globules are based on examination of at least 200 globules in several or many replicas from each of three or more labeling experiments for each antigen. Owing to the paucity of mammary cells in the milk supernatants, it was possible to evaluate mammary epithelial cells only in experiments localizing adipophilin and butyrophilin.