Supplementary material for Quetglas et al. (2000) Proc. Natl. Acad. Sci. USA 97 (17), 9695-9700.

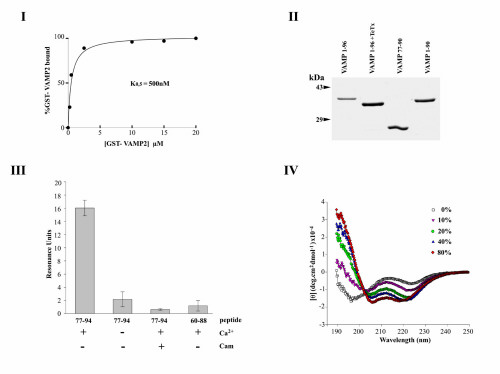
Fig. 5. (I) Saturation analysis of calmodulin binding to GST-VAMP 21-96. The indicated concentrations of GST-VAMP 2 were incubated with calmodulin-agarose in TBS in the presence of 1 mM CaCl2. After extensive washing, bound proteins were eluted with electrophoresis sample buffer and quantified by SDS/PAGE, Coomassie blue staining, and densitometry. Data showed an apparent KD = 500 nM and a stochiometry of 0.6 mol VAMP/1 mol calmodulin bound at saturation. The calmodulin concentration was determined from the supplier’s specifications. Binding activity was assumed to be 100% but is likely to be lower. Thus, in view of the single consensus calmodulin interaction site identified in the VAMP sequence, these results suggest a stochiometry of 1/1. (II) SDS/PAGE of VAMP fusion proteins. GST-fusion proteins containing the sequences VAMP1-96, VAMP1-96 cleaved with TeTx light chain (i.e., VAMP1-76), VAMP77-90, and VAMP1-90 were analyzed by SDS/PAGE and Coomassie blue staining. (III) Binding of VAMP peptides to calmodulin. The interactions of synthetic peptides VAMP77-94 and VAMP60-88 with biotinylated calmodulin immobilized on a streptavidin-coated sensor-chip were assayed by SPR, in the presence or absence of Ca2+ (0.5 mM) or soluble calmodulin (6 µM). Results (mean + SD, n = 3) indicate that residues within the sequence 89-94 play a critical role in calmodulin binding. (IV) Circular dichroism spectroscopy of the synthetic VAMP peptide 77-94. Spectroscopy was performed at 25°C in the presence of increasing concentrations of trifluoroethanol in H2O, buffered to pH 7.0 with 10 mM sodium phosphate, using a quartz cell of 1 mm path length. Data indicate that hydrophobicity induces increasing alpha-helical content.