FIGURE 1: Prevalence of H. pylori in Non-ulcer Dyspepsia
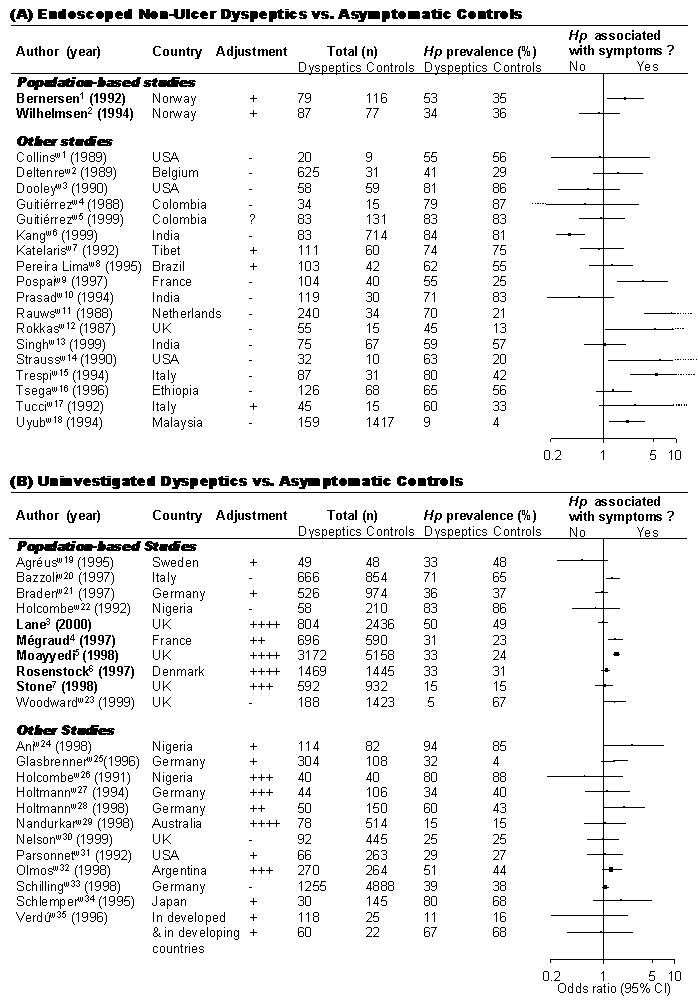
Black squares indicate odds ratios, sizes of squares are proportional to sample size, horizontal lines represent 95% confidence intervals.
Population-based studies
: patients and controls sampled from an unselected general populationOther studies:
dyspeptics and controls recruited from a pre-selected population: (i.e., blood donors, employees, etc.) or patient-based studies (dyspeptic subjects from 2nd or 3rd referral centre, controls recruited opportunistically)Only studies in adults were considered.
Degree for adjustment of confounders
: - none; + age and sex; ++ age, sex, and smoking or socio-economic status; +++ age, sex, smoking and socio-economic status; ++++ age, sex, smoking, socio-economic status, ethnicity and/or alcohol consumption.? = information not available
References for figures
FIGURE 2: Effect of H. pylori Therapy on Dyspeptic Symptoms
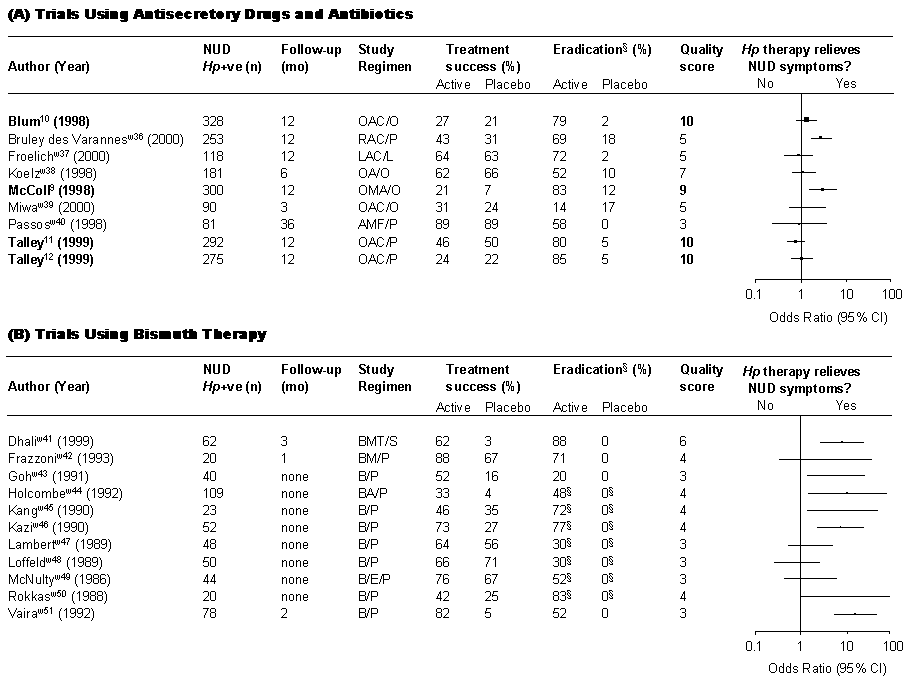
The quality of a particular trial is expressed by its score value* that includes: appropriate definition of dyspepsia (1); adequate sample size (1); random assignment to an anti-H. pylori regimen expected to achieve eradication rates in at least 90% (2); adequate blinding (1); symptoms assessed by validated questionnaires (1); duration of follow-up at least 6 months (1); endoscopy at inclusion and at follow-up (1); treatment success defined as no or minimal symptoms (1); intention-to-treat analysis (1).
Maximum score = 10.
*Number in parentheses indicates the points awarded for a particular factor.
Treatment:
A, amoxicillin; B, bismuth-based compounds (i.e. bismuth subsalicylate or colloidal bismuth citrate); C, clarithromycin; E, erythromycin; F, furozalidine; L, lansoprazole; M, metronidazole; O, omeprazole; P, placebo; R, ranitidine; S, sulfacrate; T, tetracycline.§
suppression instead of eradication of H. pylori References for figures