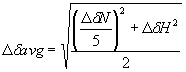
Bayliss et al. 10.1073/pnas.0609535104. |
Fig. 6. Identification of a C-terminal domain of VirB9 that is soluble, folded, and interacts with VirB7. (A) Limited proteolysis of Brucella suis VirB9 (BsB9) using Factor Xa or Enterokinase (Ek) produces two bands corresponding to residues 23-159 (BsB9NT) and 165-289 (BsB9CT). Increasing concentrations of protease yield increasing cleavage of full-length protein into two domains (gradient triangles). (B) SDS/PAGE of purified complex of BsB9CT and His6BsB7, showing 1:1 stoichiometry. From left to right, the lanes correspond to the peak fractions from a size-exclusion column. (C) SDS/PAGE of purified complex of TraOCT and TraN (Upper) and TraOCT alone (Lower). In both cases, from left to right, the lanes correspond to peak fractions from chromatographic procedures shown in D. (D) Gel-filtration traces of TraOCT in complex with TraN (Left) and alone (Right). SDS/PAGE analysis of the two peaks of oligomeric "O" and monomeric "M" TraOCT is shown in C Lower.
Fig. 7. Solution structure of TraOCT/TraN complex. (A) Overlay of the 1H,15N HSQC spectra of TraOCT in the absence (blue) and presence (red) of unlabeled TraN at 1:1 molar ratio. Selected residues that show the greatest chemical shift difference between free and bound TraO are labeled. (Inset) The ablation of cross-peak intensity for free E191 and increase of bound E191 with increasing concentration of TraN. (B) Overlay of the 1H,15N HSQC spectra of 15N-TraOCT/unlabeled TraN (red) and 15N-TraN/unlabeled TraOCT (black). (C) Superposition of the 20 best-fit NMR-derived structures for TraOCT in the complex. b-Strands are color-ramped from blue (b 1), cyan (b 2), green (b 3), lime green (b 4), yellow (b 5), orange (b 6), brown (b 7), magenta (b 8), to red (b 9).
Fig. 8. A. tumefaciens cells were reacted with anti-VirB9 antibodies and examined by immunofluorescence (IFM) (Upper) and differential interference contrast (DIC) microscopy (Lower). Strains: DB9 (PC1009); DB9(B9), PC1009 with pBLC373 producing native VirB9.
Table 1. Summary of structure statistics for TraOCT I
<SA>* | SAlowest | |
Experimental restraints† | ||
All, Å (2,457) | 0.021 ± 0.001 | 0.021 |
Intraresidue B7 (117) | 0.006 ± 0.003 | 0.006 |
Sequential B7 (111) | 0.032 ± 0.009 | 0.033 |
Short B7 (33) | 0.028 ± 0.005 | 0.024 |
Intraresidue B9 (509) | 0.009 ± 0.001 | 0.008 |
Sequential B9 (461) | 0.024 ± 0.004 | 0.026 |
Short B9 (160) | 0.017 ± 0.001 | 0.014 |
Long B9 (777) | 0.019 ± 0.001 | 0.019 |
Interprotein (235) | 0.035 ± 0.004 | 0.031 |
Hydrogen-bond restraints, Å (54) | 0.022 ± 0.008 | 0.016 |
Dihedral-angle restraints, ° (118) | 0.32 ± 0.03 | 0.32 |
Number of residual restraints violations | ||
NOE violations >0.4 Å | 0.5 ± 0.7 | 0 |
Angle violations >3° | 0 | 0 |
Deviations from idealized covalent geometry‡ | ||
Bonds, Å (1,886) | 0.0015 ± 0.0001 | 0.0015 |
Angles, ° (3,414) | 0.35 ± 0.01 | 0.36 |
Improper dihedrals, ° (998) | 0.2 ± 0 | 0.2 |
Structural statistics for the ensemble§ | ||
PROCHECK parameters | ||
Most-favored region, % | 79.3 ± 3.5 | 83.1 |
Additionally allowed, % | 18.2 ± 3.5 | 14.5 |
Generously allowed, % | 2.7 ± 1.5 | 2.4 |
Disallowed, % | 0.6 ± 0.0 | 0 |
Number of bad contacts | 1.8 ± 1.2 | 1 |
Rmsd from the average structure¶ | ||
Backbone (N, Ca, C), Å | 0.42 ± 0.06 | 0.40 |
Heavy atoms, Å | 0.88 ± 0.09 | 0.90 |
*<SA> represents the set of 20 selected conformers obtained by restrained dynamical simulated annealing in CNS/Xplor-NIH. SAlowest refers to the lowest energy structure of the set.
†
Sum averaging of NOE distance restraints was used for groups with degenerate proton chemical shifts. The interproton unambiguous distance restraint list comprised 626 intraresidue, 572 sequential (|i - j|= 1|), 193 short range (1<|i - j|<5), and 1012 long-range (|i - j|>5). Hydrogen-bonds restraints were applied as pairs of distance restraints: HN•••O, 1.2-2.2 Å; N•••O, 1.2-3.2 Å. The final values for the respective force constants were: NOE, 30 kcal mol-1 Å-2; H-bonds, 50 kcal mol-1 Å-2; dihedral angles, 200 kcal mol-1 rad-2.‡
The final values for the respective force constants were: bond lengths, 1,000 kcal mol-1 Å-2; angles and improper torsions, 500 kcal mol-1 rad-2; the improper torsion angle restraints serve to maintain planarity and chirality.§
The program PROCHECK (21) was used to assess the stereochemical parameters of the family of conformers for the VirB9 moiety only. The figures indicate the percentage of residues with backbone f and y angles in separate regions of the Ramachandran plot, defined in the program. The number of bad contacts per 100 residues is expected to be in the range 0-30 for protein crystal structures of >3.0-Šresolution.¶
The precision of the atomic coordinates is defined as the average pair-wise rmsd between each of the 20 conformers and a mean coordinate structure SA generated by iterative best-fit of the backbone atoms (N, Ca, and C) over residues 180-266 of TraOCT (omitting the flexible N and C termini, and TraN), followed by coordinate averaging.Table 2. Mutational analysis of the interface between VirB7 and VirB9
Strains* | Plasmids† | VirB7 composition‡ | VirB9 composition‡ | VirB9§ | VirB7§ | VirB7-VirB7 disulfide bridge§ | VirB7-VirB9 disulfide bridge§ | VirB10 stability§ | Pilus production¶ | Virulence on plants | Conjugative transfer of IncQ plasmid |
A348 (WT) |
|
|
| + | + | + | + | + | ++ | ++ | +++ |
PC1007 |
| D virB7 |
| - | - | - | - | - | - | - | - |
PC1009 |
|
| D virB9 | - | + | + | - | - | - | - | - |
PC1007 | pBLC974 | VirB7 WT | VirB9 WT | - | + | + | - | - | - | - | - |
PC1007 | pXZB14 | VirB7 C24S | VirB9 WT | - | - | - | - | - | - | - | - |
PC1007 | pBLC1 | VirB7 I28A, F29A | VirB9 WT | - | + | +/- | - | - | - | - | - |
PC1007 | pBLC2 | VirB7 L31A, N32A | VirB9 WT | - | + | +/- | - | - | - | - | - |
PC1007 | pBLC3 | VirB7 I28A, F29A, N32D | VirB9 WT | - | + | +/- | - | - | - | - | - |
PC1009 | pBLC373 | VirB7 WT | VirB9 WT | + | + | + | + | + | + | + | + |
PC1009 | pXZB21 | VirB7 WT | VirB9 C262S | - | + | - | - | - | - | - | - |
PC1009 | pBLC4 | VirB7 WT | VirB9 A191M | - | + | +/- | - | - | - | - | - |
PC1009 | pBLC5 | VirB7 WT | VirB9 V202W | + | + | +/- | + | + | + | - | - |
PC1009 | pBLC6 | VirB7 WT | VirB9 L198F, V202W | - | + | +/- | - | - | - | - | - |
PC1009 | pBLC7 | « | VirB9 F203W | - | + | +/- | - | - | - | - | - |
*PC1007 and PC1009 are derivatives of WT strain A348 with DvirB7 or DvirB9 mutations, respectively, on the pTiA6NC plasmid.
†
Plasmid sources: pXZB14 and pXZB21 (1); pLL9196 and pLL9206 (19). Other plasmids were constructed in this study.‡
The listed plasmids produce the VirB7 and VirB9 WT or mutant proteins presented in bold.§
Detection of VirB proteins by Western blotting and immunodetection using corresponding anti-VirB antiserum.¶
Pilus production was monitored by accumulation of exocellular VirB2 pilin (19).19. Jakubowski SJ, Cascales E, Krishnamoorthy V, Christie PJ (2005) J Bacteriol 187:3486-3495.
SI Text
Supporting Results
Identification of a Soluble, C-Terminal VirB7-Interacting Domain of VirB9.
We first expressed Brucella suis VirB9 (BsB9) lacking its periplasmic targeting sequence and then used limited proteolysis, mass spectrometry, and N-terminal sequencing to define a soluble fragment suitable for structural studies (SI Fig. 6A). Full-length or N-terminal (residues 23-159) BsB9 were not stable in solution, but a C-terminal fragment (VirB9CT, residues 165-289) was stable. We also produced a similar C-terminal fragment of the VirB9 homologue from pKM101 TraO (TraOCT; residues 177-294) that was also found to be stable.Previous work suggested that the interaction between VirB7 and VirB9 homologues occurs between VirB7 and the C-terminal region of VirB9 (1-4). For this study, we expressed soluble versions of VirB7 that lacked lipidation (see Supporting Methods, below, for details). We were unable to form a complex between Brucella suis VirB9CT and VirB7 except when we coexpressed the two proteins (SIb Fig. 6B). Unfortunately, this complex did not crystallize. We then focused our attention on the corresponding proteins from pKM101 TraO (TraOCT residues 177-294) and TraN (residues 17-49). A complex between the two proteins formed after expression and purification of the proteins separately or after coexpression and copurification (SI Fig. 6C Upper). We were also unable to obtain crystals of the pKM101 proteins. We therefore turned to NMR to solve the structure of these complexes. NMR spectroscopy provides a means to investigate the interactions of macromolecules. More specifically, titrations of a potential binding partner with an isotope-labeled target monitored by 2D heteronuclear spectroscopy are commonly used to identify the binding surface. However, to perform 1H-15N HSQC NMR experiments in which 15N-labeled VirB9 is titrated with unlabeled VirB7 requires the ability to express 15N-labeled VirB9 separately and to form the complex from the purified proteins. Because this was achieved only for the TraOCT-TraN complex, we determined the NMR structure of this complex.
NMR Structure Determination.
We purified 15N-labeled TraOCT alone and bound to unlabeled TraN. In contrast to the complex, which eluted from the gel filtration column as a single peak corresponding to the correct heterodimer size, TraOCT alone eluted mainly as two peaks, corresponding to a monomer and a dimer, with a small percentage of aggregated material (SI Fig. 6D). The peak fractions were analyzed by 1D 1H NMR separately and, although the spectrum of the dimeric component suffered from peak broadening, the spectrum of the TraOCT monomeric species is indicative of a small protein with a globular fold (data not shown). Over a period of ≈1 week, the characteristics of the 1H NMR spectrum of the monomer sample changed to appear more like the dimer spectrum (data not shown), indicating a slow transition to a higher oligomeric state.We observed that when TraOCT interacts with TraN, residues at the binding interface experience a differing chemical environment, resulting in a chemical shift change (Dd) of the cross-peak in the 1H-15N HSQC spectrum. Comparison of the 1H-15N HSQC spectra of 15N-labeled TraOCT alone or in a 1:1 molar ratio with unlabeled TraN reveals binding of TraN to TraOCT (SI Fig. 7A). Binding of TraN is characterized by a concomitant decrease in the cross-peak intensity for free TraOCT, with an increase in the cross-peak intensity for bound TraOCT, indicating slow exchange on the NMR time scale, and a slow off-rate, suggesting relatively strong binding. The 1H-15N HSQC spectrum of 15N-labeled TraN (36 residues) in complex with unlabeled TraOCT shows chemical shift dispersion that is clearly consistent with an ordered conformation (SI Fig. 7B).
Samples of either 15N- or (13C,15N)-labeled TraOCT/unlabeled TraN or unlabeled TraOCT/15N- or (13C,15N)-labeled TraN were prepared, and full resonance assignments were obtained (5). On the basis of the analysis of 3D 15N- and 13C-separated 1H-NOESY (Nuclear Overhauser Effect Spectroscopy) spectra, we determined the 3D solution structure of the complex between TraOCT and TraN. A total of 2,403 NOE-derived interproton distance restraints for TraOCT/TraN, including 235 interprotein TraOCT-TraN NOEs, were used in the final iterations of the structure calculations for residues 177-271 of TraOCT and 21-42 of TraN (SI Table 1). Residues 17-20 and 43-48 of TraN and 272-294 of TraOCT were omitted from the structure calculations because they displayed low 1H-15N heteronuclear NOE values (<0), indicating internal flexibility on a fast time scale (data not shown). Omitting a small number of residues at both the N and C termini of TraOCT, the refined conformer bundles represent well defined models for the TraOCT domain with rmsd values of 0.42 ±0.06 Å for the backbone and 0.88 ± 0.09 Å for all of the heavy atoms (residues included in the superposition are 180-266; SI Fig. 7C). TraN has a Ca RMSD of 2Å between the 20 members of the ensemble. Two stretches of the polypeptide chain show a Ca RMSD below 1Å, between Pro-23 and Trp-27 and between Thr-30 and Asn-34 (Fig. 2). These stretches form the main interaction site with TraOCT (Fig. 1).
Supporting Methods
Protein Preparation.
StrepII-tagged B. suis VirB9 protein [residues 19-289, lacking the N-terminal periplasmic localization signal; gift of Dr. Christian Baron (McMaster University, Ontario, Canada)], was expressed in Codonplus RPIL (ArgProIleLeu) Escherichia coli (Stratagene) and purified by using a streptactin Sepharose column (IBA). Limited proteolysis was carried out over 16 h at 4°C by using Factor Xa (Qiagen) and Enterokinase proteases. N-terminal sequencing was carried out by Midwest Analytical Inc. (St. Louis, MO). Mass spectra were collected on a Micromass Platform single-quadrupole mass spectrometer (Waters Ltd), and spectra were processed by using Masslynx software.BsB7 (residues 17-58) and BsB9CT (residues 165-289) were cloned into the first and second multiple-cloning sites of pRSF-Duet (Novagen), respectively, and coexpressed in Codonplus RPIL E. coli (Stratagene). The complex was purified by using a Ni column (His-Trap), followed by gel filtration.
The sequence encoding TraN lacking its periplasmic localization and lipid-modification signals (residues 17-49) was cloned in pGEXcs and expressed in Codonplus RPIL E. coli (Stratagene) as a TEV (Tobacco Etch Virus) protease-cleavable GST-fusion. The sequence encoding TraOCT (residues 177-294) was cloned in pET151-TOPO and was expressed in Codonplus RPIL E. coli (Stratagene) as a TEV-cleavable His6 fusion protein. TraOCT was purified by using a Ni column (His-Trap), followed by TEV protease cleavage, removal of TEV protease and His tag by a Ni column, and gel filtration. TraOCT eluted from the gel filtration as two peaks (SI Fig. 6D), which were separately pooled for NMR spectroscopy. A complex between TraOCT and TraN was purified by mixing E. coli cell lysates expressing the individual proteins and passing them first through a Ni column (His-Trap) and then binding to a GST-Trap column. The complex was eluted from the GST-Trap column by TEV protease cleavage, and TEV protease and the His tag were removed by a second His-Trap column. The complex eluted as a single peak by gel filtration (SI Fig. 6D). For NMR spectroscopy, protein samples were prepared in a buffer containing 10 mM Na/K phosphate, pH 6.7, 100 mM NaCl, 1 mM EDTA, and 0.2% sodium azide. 15N- or 13C-labeled proteins were produced by growing E. coli in minimal media supplemented with 15N ammonium sulfate (Sigma) or 13C D-glucose (Sigma).
NMR Spectroscopy.
NMR spectra were acquired at 298 K (except where indicated) on Varian UnityPLUS and UnityINOVA spectrometers (operating at nominal 1H frequencies of 500, 600, and 800 MHz), each equipped with a triple resonance probe, including a z axis pulse field gradient coil. Sequence-specific resonance assignments were obtained by using standard triple resonance NMR spectroscopy as described in Harris et al. (5). Distance restraints were derived from 3D 15N- and 13C-edited NOESY-HSQC spectra with a mixing time of 120 ms. {1H}15N heteronuclear NOE data for both 15N-labeled TraN/unlabeled TraOCT, and 15N-labeled TraOCT/unlabeled TraN were recorded with 3.0 sec of 1H saturation in the latter part of a 3.5-sec preparation delay, which was also used without radio frequency pulses for the reference 2D spectrum without NOE. Residues 17-20 and 43-48 of TraN and 272-294 of TraOCT displayed {1H}15N heteronuclear NOE values <0, indicating internal flexibility on the NMR timescale. All NMR spectra were processed by using NMRpipe/NMRDraw (6) and analyzed by using ANSIG for openGL v1.0.3 (7). 1H, 13C, and 15N chemical shifts were referenced indirectly to sodium 2,2-dimethyl-2-silanepentane-5-sulfonate (DSS), by using absolute frequency ratios for the 1H signals (8). The interaction titration of TraOCT domain with TraN was performed at a constant concentration of TraOCT by using the method described by McAlister et al. (9), ranging from TraOCT:TraN molar ratios 1:0 to 1:1. Any changes in the spectrum of the labeled component during the titration can be attributed directly to an intermolecular interaction, because both proteins are preexchanged into the same stock buffer. Average chemical shift changes for the backbone N and HN atoms of the VirB9CT domain were calculated by using the equation below, where DdN represents the change in amide nitrogen's chemical shift, and DdH represents the change in the amide proton's chemical shift.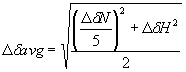
Sequence-specific resonance assignment of TraOCT in the absence of TraN was obtained by using standard triple resonance experiments, namely: HNCA, HN(CO)CA, HNCACB, CBCA(CO)NH, HA(CA)NH, and HA(CACO)NH. By using the 13Ca, 13Cb, 13CO, and 1Ha chemical shift values, TraOCT in the absence of TraN is predicted by the Chemical Shift Index method (10) to have the same secondary structure elements as TraOCT in complex with TraN.
Structure Calculations.
Interproton distance restraints were derived from the ANSIG cross-peaks file of 3D 15N-NOESY-HSQC and 13C-NOESY-HSQC spectra for TraOCT/TraN. A proportion of the resonances were successfully assigned in a manual fashion without ambiguity. The remaining cross-peaks appearing at positions in the spectrum with overlapping resonances were labeled with ambiguous assignments by reference to the chemical shift list obtained with through-bond correlation spectra, by using the "Connect" module from the program AZARA (11). The cross-peaks were grouped into four categories according to their relative peak intensities: strong, medium, weak, and very weak and were designated with the corresponding interproton distance restraint limits of 1.8-2.5 Å, 1.8-3.0 Å, 1.8-3.5 Å, and 1.8-4.0 Å, respectively. A 0.5-Å per methyl group was added to the upper bound of the distance restraint for NOE cross peaks that involved methyl groups.All structures for TraOCT/TraN were calculated by using an ab initio simulated annealing protocol within the CNS program (12), with PARALLHDGv5.3 force field and PROLSQ nonbonded energy function (13). The protocol adopts a mixture of Cartesian molecular dynamics (MD) and torsion angle dynamics-simulated annealing to refine structures starting from random-generated conformers with good local geometry.
A total of 2403 NOE-derived interproton distance restraints for TraOCT/TraN were included in the final iterations of the structure calculations (see SI Table 1). Backbone torsion angle restraints for j and y were derived from analysis of 1Ha, 13Ca, 13Cb, 13C', and 15NH chemical-shift databases as implemented in the program TALOS (14). Hydrogen-bond restraints for amide protons were derived from an assessment of the regular secondary structure elements. A total of 118 dihedral angle and 54 hydrogen bond (27 H-bonds; 2 distance restraints per H bond) interatomic distance restraints were used for TraOCT/TraN.
The atomic coordinates of the final 20 simulated annealing TraOCT/TraN conformers and the list of experimental restraints (PDB ID code 2OFQ) has been deposited at the RCSB Protein Data Bank. Chemical shifts for resonance assignments for the TraOCT/TraN complex have been deposited at the BioMagResBank (accession code 6936).
A. tumefaciens
Strains and Plasmids. A. tumefaciens A348 served as the wild-type strain. PC1009, PC1007, and PC1000 are A348 derivatives deleted of virB9, virB7, and all virB operon, respectively (15, 16). Conditions for growth of A. tumefaciens and E. coli and for vir gene induction with 200 mM acetosyringone in induction medium have been described (15). Plasmids used in these studies were constructed as described below.Protein Analyses and Immunodetection.
SDS/PAGE using glycine or tricine buffer systems, Western blotting, and immunostaining with anti-VirB antibodies was performed as described (17). Polyclonal antiserum specificities were previously documented for VirB7 (16) and VirB9 (18).Virulence and Conjugation Assays.
Virulence assays on Kalanchoe daigremontiana leaves were carried out at least three times with three independent bacterial cultures, as described (15). Conjugative transfer of the mobilizable plasmid pML122 (IncQ) to A. tumefaciens recipient strain A348 Spcr was carried out as described (19).virB9
Gene Mutants Constructions. The pBLC373 vector is pJW373 (18) ligated into the broad host range vector (BHR) pBBRMCS. Plasmid pBLC5, producing VirB9 with a V202W substitution mutation, was constructed by QuikChange mutagenesis (Stratagene) using pJW373 as the template and mutagenic oligonucleotides VirB9V202Wc 5'-CCACTCGAATGGTTCGACAAC-3' and VirB9V202Ww 5'-GTTGTCGAACCATTCGAGTGG-3'. Plasmids pBLC4 and pBLC6, producing VirB9 with A191M and L198F/V202W mutations, respectively, were constructed by the megaprimer method using pJW373 as the template. For pBLC4, the mutagenic primers VirB9A191Mc 5'-TGGCACTACGTCATGCAAGGCGATCGT-3' and the VirB10D27-46 5'-GTCATTCACCTTCTTTTGACGCCCAC-3' were used to synthesize a 494-bp PCR fragment that then served as a primer for a second PCR with the M13 reverse primer 5'-GGAAACAGCTATGACCATG-3'. The mutated PCR fragment was inserted into pCRII-TOPO, which was then ligated to a BHR vector to yield pBLC4. The mutagenic primers VirB9V202Wc and the VirB10D27-46 were used to synthesize a 458-bp PCR fragment that then served as a primer for a second PCR with the M13 reverse primer. The mutated PCR fragment was inserted into pCRII-TOPO, and sequencing revealed V202W as well as a second mutation, L198F. This plasmid was ligated to a BHR vector to yield pBLC6.Cys substitution mutations in VirB9 were generated by QuikChange (Stratagene) or the Kunkel method (20) using pJW373 as a template. For pBALM1 producing VirB9N216C, the mutagenic oligonucleotides were VirB9N216Cs 5'-CTTTCCCGGGTGCGTACGCATACCC-3' and VirB9N216Cas 5'-GGGTATGCGTACGCACCCGGGAAAG-3'. For pBALM2 producing VirB9N226C, the mutagenic oligonucleotides were VirB9N226Cs 5'-CTACACCATCTGCCCTGACGGCAAGGAAGC-3' and VirB9N226Cas 5'-GCTTCCTTGCCGTCAGGGCAGATGGTGTAG-3'. For pBALM4 producing VirB9D228C, the mutagenic oligonucleotides were VirB9D228Cs 5'-CATCAATCCTTGCGGCAAGGAAGCTGTC-3' and VirB9D228Cas 5'-GACAGCTTCCTTGCCGCAAGGATTGATG-3'. For pBALM3 producing VirB9P227C, ssDNA prepared from pJW373 served as the template together with the mutagenic oligonucleotide VirB9227Cas 5'-GCTTCCTTGCCGTCGCAATTGATGGTG-3' according to Kunkel (20)
virB7
Gene Mutants Constructions. Plasmid pPC974 was ligated to pXZ151 to yield plasmid pLC974. Plasmid pBLC1, producing a VirB7 variant with I28®A and F29®A substitution mutations and plasmid pBLC2, producing VirB7 with L31®A and N®32A mutations, were constructed by the megaprimer method using pPC974 as the template. For pBLC1, VirB7IFAAc 5'-AGCGAGCTGCAAAGGCCCGGCAGCACCGCTGAAT-3' and the M13 reverse primer were used to synthesize a 360-bp PCR fragment that then served as a primer for a second PCR reaction with the M13 forward primer 5'-TGTAAAACGACGGCCAGT-3'. The mutated PCR fragment was inserted into pCRII-TOPO, which was then ligated to a BHR vector to yield pBLC1. The sequencing of another clone from the initial pCRII-TOPO ligation revealed an extra point mutation substituting N32®D, yielding plasmid pBLC3 after ligation into the BHR vector. For pBLC2, VirB7LNAA 5'-TTTCCCGGCCGCTGTGGGGCGATGG-3' and the M13 reverse primer were used to synthesize a 339-bp PCR fragment that then served as a primer for a second PCR with the M13 forward primer. The mutated PCR fragment was inserted into pCRII-TOPO, which was then ligated to a BHR vector to yield pBLC2.1. Spudich GM, Fernandez D, Zhou XR, Christie PJ (1996) Proc Natl Acad Sci USA 93:7512-7517.
2. Fernandez D, Spudich GM, Zhou XR, Christie PJ (1996) J Bacteriol 178:3168-3176.
3. Baron C, Thorstenson YR, Zambryski PC (1997) J Bacteriol 179:1211-1218.
4. Anderson LB, Hertzel AV, Das A (1996) Proc Natl Acad Sci USA 93:8889-8894.
5. Harris R, Bayliss R, Waksman G, Driscoll PC (2006) J Biol NMR 36:31.
6. Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) J Biomol NMR 6:277-293.
7. Kraulis PJ (1989) J Magn Reson 24:627-633.
8. Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD (1995) J Biomol NMR 6:135-140.
9. McAlister MS, Mott HR, van der Merwe PA, Campbell ID, Davis SJ, Driscoll PC (1996) Biochemistry 35:5982-5991.
10. Wishart DS, Sykes BD (1994) J Biomol NMR 4:171-180.
11. Boucher W (1996) AZARA v 2.7 (Department of Biochemistry, University of Cambridge, Cambridge, UK)
12. Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. (1998) Acta Crystallog D 54:905-921.
13. Linge JP, Nilges M (1999) J Biomol NMR 13:51-59.
14. Cornilescu G, Delaglio F, Bax A (1999) J Biomol NMR 13:289-302.
15. Berger BR, Christie PJ (1994) J Bacteriol 176:3646-3660.
16. Fernandez D, Dang TA, Spudich GM, Zhou XR, Berger BR, Christie PJ (1996) J Bacteriol 178:3156-3167.
17. Jakubowski SJ, Krishnamoorthy V, Christie PJ (2003) J Bacteriol 185:2867-2878.
18. Ward JEJ, Dale EM, Christie PJ, Nester EW, Binns AN (1990) J Bacteriol 172:5187-5199.
19. Jakubowski SJ, Cascales E, Krishnamoorthy V, Christie PJ (2005) J Bacteriol 187:3486-3495.
20. Kunkel TA, Bebenek K, McClary J (1991) Methods Enzymol 204:125-139.
21. Laskowski RA, Moss DS, Thornton JM (1993) J Mol Biol 231:1049-67.