Blood, Vol. 109, Issue 8, 3538-3543, April 15, 2007
Pathogenic proline mutation in the linker between spectrin repeats: disease caused by spectrin unfolding
Blood Johnson et al. 109: 3538
Supplemental materials for: Johnson et al, Vol 109, Issue 8, 3538-3543
Files in this Data Supplement:
- Document 1. Supplemental methods and results (PDF, 83.8 KB)
- Figure S1. Thermal denaturation studies of the WT and Q471P mutant (JPG, 30.3 KB) -
(A) Tryptophan fluorescence and (B) circular dichroism versus tryptophan fluorescence. This additional method shows significant thermal destabilization of the Q471P mutant relative to the WT construct at or below 37°C. The loss of tertiary (tryptophan fluorescence) without corresponding loss in secondary structure (CD) has been observed in other studies of recombinant proteins and is typical of the molten globule state, which is often described as having a well-defined secondary structure without specific tertiary structure. The helicity plot against tryptophan fluorescence data at indicated temperatures is taken from Figure 2.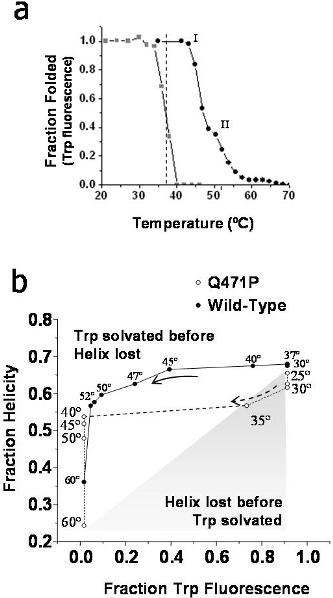
- Figure S2. Isothermal calorimetry results tabulated for the recombinant peptides at 23°C (JPG, 61.3 KB) -
At 37°C, the Kd increased essentially the same, to approximately 8 µM for WT and 6.8 µM for the Q471P mutant (n = 1.1 for both), which is a 15- to 18-fold weaker interaction. For full-length spectrin heterodimer associating into tetramer, a 7-fold decrease in affinity has been reported over a similar temperature range. The heterotetramer thus appears more thermally stable than the heterodimer.