Pohl et al. 10.1073/pnas.0701425104. |

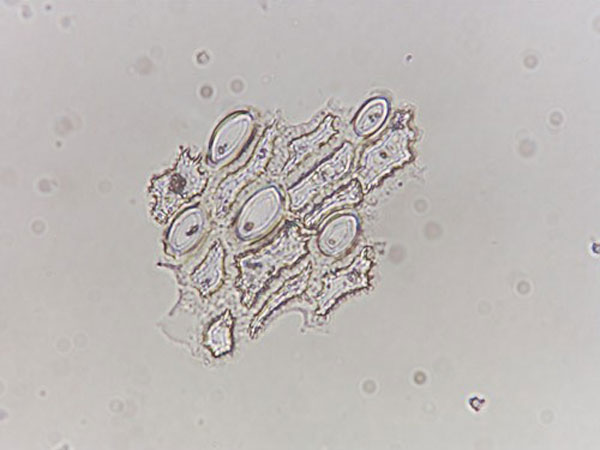
Fig. 4. A group of phytoliths still articulated as they would occur in living plant tissue removed from the outer glume of a Balsas teosinte fruitcase. Phytolith formation occurs in both long cells and short cells, and all types of phytoliths are highly decorated.

Fig. 5. Phytoliths from the glumes and cupules of a maize cob (the race Amarillo). Unlike in teosinte, the phytolith assemblage is dominated by rondels, and no long cell phytoliths are present. The rondels are also less highly decorated around their edges than those in teosinte.
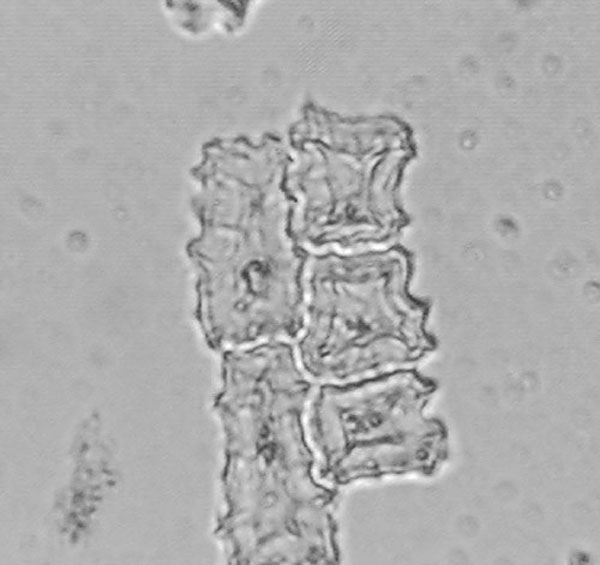
Fig. 6. A group of long cell phytoliths removed from the fruitcase of Tripsacum dactyloides. They have serrated edges and other morphological characteristics that make them distinct from wild and domesticated Zea and unique to the genus Tripsacum.

Fig. 7. Laminations in an Archaic Period section of the SAV-4 vibracore from San Andrés, Tabasco, Mexico (core depth 800 cm). Sediments contained maize pollen.
Figs. 8.
(a and b) Location of significant sites (discussed in the text) with the earliest currently available microfossil evidence for maize dispersals out of Mexico through Central America and into northern and then southern South America. The dates, sites, and type of evidence are explained below. We note that the record of early maize may be uneven, a result of several non-mutually exclusive factors. For example, lakes and swamps are discontinuous over the landscape and when studied, fail to record activity beyond their immediate watersheds. Systematic archaeological research directed toward locating and investigating human occupations dating to the relevant time periods (8,000-6,000 cal BP) has yet to be carried out in many regions. Furthermore, maize pollen is heavy and often deposited close to its source, i.e., <75 m away (1). Thus, there is a statistical chance that in any paleoecological sequence the earliest maize cultivators have eluded detection, especially if agricultural plots were located away from lake shores and swamp margins.El Venancio, Río Balsas watershed, Mexico. Maize pollen and vegetational disturbance indicating slash and burn cultivation at the base of this paleoecological sequence dated to 4500 cal BP (2). Data such as these on ancient agriculture and associated deforestation in the poorly studied, deciduous tropical forests of maize's postulated homeland are just emerging.
Highland Western Panama. Maize starch grains on plant grinding stones and other artifacts from archaeological sites by 7000 cal BP (sites: Hornito and Trapiche) (3).
Central Pacific Panama. Maize cob and leaf phytoliths and maize starch grains in archaeological sediments and on plant grinding stones and other artifacts by 7,800 cal BP (sites: Cueva de los Ladrones, Aguadulce Rock Shelter, Los Santanas); maize pollen in archaeological (Cueva de los Ladrones) and paleoecological (Lake La Yeguada) sediments by 7,800 cal BP (3-6).
Cauca Valley, Colombia. Maize pollen in a paleoecological sequence from the Hacienda El Dorado by 7,800 cal BP. Maize pollen at the nearby Hacienda Lusitania before 5,800 cal BP (7).
Colombian Amazon. Maize pollen and vegetational disturbance indicating slash and burn cultivation at the site of Abeja before 5,800 cal BP (8).
Southwestern Ecuador. Maize leaf phytoliths in archaeological sediments by 7,500 cal BP at the type site of the Vegas culture (3, 4, 8). Maize cob and leaf phytoliths and maize starch grains in early ceramic (Valdivia culture) archaeological sediments and on plant grinding stones by 6,100 cal BP (sites: Loma Alta and Real Alto) (10-13).
Ecuadorian Amazon. Maize pollen and phytoliths and vegetational disturbance indicating slash and burn cultivation at Lake Ayauchi at 6,000 cal BP (14, 15).
Southern Peruvian Andes. Maize starch grains and phytoliths in archaeological sediments and on plant grinding stones at the site of Waynuna at 4,000 cal BP (16).
Southern Uruguay. Maize cob and leaf phytoliths and maize starch grains from archaeological sediments and on plant grinding stones from village mound complexes at 4,700 cal BP (17).
3. Dickau R, Ranere AJ, Cooke RG. (2006) Proc Natl Acad Sci USA 104:3651-3656..
4. Piperno DR (2006a) Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists (AltaMira Press, Lanham MD).
5. Piperno DR, Pearsall DM (1998) The Origins of Agriculture in the Lowland Neotropics (Academic, San Diego).
6. Piperno DR, Ranere AJ, Holst I, Hansell P (2000) Nature 407:894-897.
7. Bray WB, Herrera L, Cardale Schrimpff M, Botero P, Monsalve JG (1987) in Pre-Hispanic Agricultural Fields in the Andean Region, eds Denevan WM, Mathewson K, Knapp G (Brit Arch Rep Internatl Ser 359, Oxford, U.K.), pp 443-481.
8. Mora SC, Herrera LF, Cavelier I, Rodríguez C (1991) Cultivars, Anthropic Soils and Stability (Univ Pittsburgh Lat Am Arch Rep 2. Univ Pittsburgh Dept Anth).
9. Stothert K, Piperno DR, Andres TC (2003) Q Intern 109-110, 23-43.
10. Pearsall, DM (1978) Science 199:177-178.
11. Pearsall DM. (2000) Paleoethnobotany: A Handbook of Procedures, 2nd Ed (Academic, San Diego).
12. Pearsall DM, Chandler-Ezell K, Zeidler JA (2004) J Arch Sci 31:423-442.
13. Perry L, Dickau R, Zarrillo S, Holst I, Pearsall D, Piperno DR, Berman MJ, Cooke RG, Rademaker K, Ranere AJ, Raymond JS, Sandweiss DH, Scaramelli F, Table K, Zeidler JA (2007) Science 315:986-988..
14. Bush MB, Piperno DR, Colinvaux PA (1989) Nature 340:303-305.
15. Piperno DR (1990) J Arch Sci 17:665-677.
16. Perry L, Sandweiss DH, Piperno DR, Rademaker K, Malpass MA, Umire A, de la Vera K (2006) Nature 440:76-79.
17. Iriarte J, Holst I, Marozzi O, Listopad O, Alonso E, Rinderknecht A, Montaña J, (2004) Nature 432:614-617.
SI Text
Materials and Methods.
Sediments were processed for phytoliths by standard techniques (1) involving sediment dispersion in sodium bicarbonate, followed by carbonate removal with dilute HCl, organic/humic removal with concentrated HNO3/KClO3 and 10% KOH, and phytolith recovery by flotation with CdI2/KI at a density of 2.3. Counts were based on a sum of at least 100 phytoliths; the entire slides were scanned afterward to search for presence of less commonly occurring phytoliths and confirm trends computed from the percentage counts. Phytoliths were mounted in permount. Modern phytolith assemblages constructed from various kinds of tropical forests, savannas, and slash and burn agricultural plots were also compared with fossil phytolith assemblages to identify ancient vegetational associations and human land usage (1-3).Phytolith Morphology.
Maize and teosinte form significantly different kinds of phytoliths in their reproductive structures, a process controlled largely by the genetic locus tga1. In teosinte, tga1 underwrites a more extensive silicification of the epidermis of the glume and rachid (the structure homologous to the maize cupule), resulting in phytolith formation in both the long and short epidermal cells (Fig. 3). In contrast, the maize allele of this genetic locus, a product of artificial selection, directs phytoliths to be formed nearly exclusively in the short epidermal cells of cupules and glumes (1, 4) (Fig. 4).The circular to oval short cell phytoliths that dominate phytolith assemblages of maize cobs are collectively called "rondels." In teosinte, there are many highly decorated elongated and irregularly shaped long cell phytoliths as well as rondels (Fig. 2) (e.g., refs. 1, 5-9) (Figs. 3 and 4). Comparative work on the genus Zea and non-Zea grasses from the Americas shows that rondels produced in teosinte are more highly decorated on their edges than those in maize, another product of more extensive lignification in the wild plants. Furthermore, a type of rondel called "wavy top" has been found only in maize; another type called the "ruffle top" rondel appears unique to the genus Zea (1, 5-8). Thus, a genetic locus that accounted for crucial phenotypic changes during maize domestication, the reduction of the cupulate fruitcase resulting in naked, more easily processed grains and glume softening from lignin reduction, also coded for significant differences in the number and morphology of phytoliths in the cereal and its wild ancestor.
1. Piperno DR (2006) Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists (AltaMira Press, Lanham MD).
2. Piperno DR (1988) Phytolith Analysis: An Archaeological and Geological Perspective (Academic, San Diego).
3. Piperno DR, Jones JG (2003) Q Res 59:79-87.
4. Dorweiler JE, Doebley J (1997) Am J Bot 84:1313-1322.
5. Bozarth SR (1993) Plains Anth 38:279-286.
6. Iriarte J, Holst I, Marozzi O, Listopad O, Alonso E, Rinderknecht A, Montaña J, (2004) Nature 432:614-617.
7. Pearsall DM, Chandler-Ezell K, Chandler-Ezell A (2003) J Arch Sci 30:611-627.
8. Piperno DR, Pearsall DM (1993) J Arch Sci 20:337-362.
9. Mulholland SC (1993) in Current Research in Phytolith Analysis: Applications in Archaeology and Paleoecology eds Pearsall DM, Piperno DR (MASCA Res Pap Sci Arch 10, Univ Mus Arch Anth Univ Penn, Philadelphia), pp 131-145.